WORLD TRADE ORGANIZATION
Home | About WTO | News & events | Trade topics | WTO membership | Documents & resources | External relations
Contact us | Site map | A-Z | Search
español français

Discussion on the extension of COVID-19 IP waiver
At MC12, trade ministers adopted the Ministerial Decision on the TRIPS Agreement, which gives members greater scope to take direct action to diversify production of COVID-19 vaccines and to override the exclusive effect of patents through a targeted waiver over the next five years. It addresses specific problems identified during the pandemic and aims to help diversify vaccine production capacity. It also contains a commitment that no later than six months from the date of the decision (17 June), members will decide on its possible extension to cover the production and supply of COVID-19 diagnostics and therapeutics.
Many members took the floor to welcome the successful outcome at MC12, saying it proved that WTO members can put aside differences and work together to respond to the most urgent health challenges.
A group of developing members who support an extension of the waiver to cover COVID-19 diagnostics and therapeutics circulated a proposal at the meeting including an indicative timeline for the TRIPS Council's next steps in this regard.
These members argued that the waiver on COVID-19 vaccines falls short of their expectation and is not enough to help developing countries comprehensively address current and future health challenges. Equitable access to therapeutics and diagnostics, as pointed out by the World Health Organization (WHO), is critical in helping detect new cases and new variants. They said this waiver extension needs to be discussed with a sense of urgency given the fact that many least developed countries (LDCs) lack access to life-saving drugs and testing therapeutics.
Many developing countries supported the initiative. They highlighted the joint statement made by the three Director Generals of the WHO, the World Intellectual Property Organization (WIPO) and the WTO in June 2021 reaffirming their commitment to intensifying cooperation in support of access to medical technologies worldwide to tackle the COVID-19 pandemic, including vaccines, therapeutics and diagnostics. There was also a shared view that the negotiation process for the waiver extension should be open, inclusive and transparent.
Other members cautioned that more time was needed to conduct domestic consultations on a possible extension of the waiver to therapeutics and diagnostics. Some members also flagged the importance of an evidence-based negotiation as there was no evidence that intellectual property did indeed constitute a barrier to accessing COVID-19 vaccines. Some also reiterated the need for members to fully make use of all the flexibilities that already exist in the TRIPS Agreement (including compulsory licensing) before requesting new flexibilities.
The chair, Ambassador Lansana Gberie (Sierra Leone), asked members that were ready to engage to commence discussing this matter in various configurations. He encouraged members to individually report on progress to the General Council meeting on 25-26 July while some members may need more time to deliberate on the matter, he noted. The chair will inform members how best to structure discussions on this matter going forward, he added.
Members also agreed to continue exchanges under the agenda item of IP and COVID-19 so that the TRIPS Council can keep abreast of new IP measures in relation to COVID-19 and share relevant experience. The Council also decided that the Secretariat will continue compiling and updating all COVID-related IP measures in its document “ COVID-19: Measures regarding Trade-Related Intellectual Property Rights ” to serve as the basis for members' exchanges.
Members noted that this exercise is also in line with the Ministerial Declaration on the WTO Response to the COVID-19 Pandemic and Preparedness for Future Pandemics which provides for ongoing analysis of lessons learned and challenges experienced during the COVID-19 pandemic within the relevant WTO bodies.
IP and innovation: IP licensing opportunities
Under an item on IP and Innovation which had been requested by Australia, Canada, the European Union, Hong Kong China, Japan, Singapore, Switzerland, Chinese Taipei, the United Kingdom and the United States, the co-sponsors presented their new submission with a focus on IP licensing opportunities ( IP/C/W/691 , circulated on 23 June).
The co-sponsors highlighted several major ways owners of IP assets can secure a broader reach for their products and services through licensing agreements, which enable IP owners to allow the licensee to make or sell the invention during the licence period. This includes licensing of patents, copyright, trademarks and know-how.
The proponents shared experiences on how to apply different licensing models and build up a friendly ecosystem to foster IP trading. To overcome the knowledge gap and complexity of implementing IP licensing, these countries have developed various toolkits to provide training, online guidelines, contract templates, legal services and dispute settlement so that small businesses and individuals can effectively participate in IP partnerships.
Members welcomed the discussion on IP innovation and IP licensing, with some sharing their domestic practices. WIPO introduced its recent activities in support of IP licensing, including the establishment of an IP and innovation ecosystems sector, the work of the WIPO arbitration and mediation centre, and guidance to help start-ups develop their IP strategy.
Non-violation and situation complaints
WTO members welcomed the decision adopted at MC12 to extend the moratorium on non-violation and situation complaints (NVSCs) under the TRIPS Agreement until the next Ministerial Conference (MC13). The decision tasked members to continue examining possible scope and modalities for NVSCs and to make recommendations to MC13.
This concerns the longstanding issue of whether members should have the right to bring dispute cases to the WTO if they consider that another member's action or a specific situation has deprived them of an expected benefit under the TRIPS Agreement, even if no specific TRIPS obligation has been violated.
This moratorium was originally set to last for five years (1995–99), but it has been extended a number of times since then in the absence of agreement by members on what the scope and modalities could look like if non-violation and situation complaints were to apply to the TRIPS Agreement.
At the meeting, several developing countries suggested continuing the examination of the scope and modalities of such complaints, with the aim of making it applicable to WTO dispute settlement. Some members backed the idea of seeking a permanent solution on this matter while others were concerned that allowing NVSC dispute complaints might jeopardize the flexibilities granted in the TRIPS Agreement.
More information on the TRIPS non-violation issue is available here .
Technical cooperation and capacity building
WIPO briefed the meeting on the WHO-WIPO-WTO COVID-19 Technical Assistance Platform , which offers a one-stop shop to help members and WTO accession candidates address their capacity building needs to respond to the COVID-19 pandemic.
The chair urged members to submit information on their activities in technical cooperation and capacity building as well as incentives for technology transfer by 12 September in preparation for the end-of-year annual review. Members are encouraged to use the online submission system ( e-TRIPS ) to make submissions.
Other matters
The European Free Trade Association was granted observer status for the next Council meeting.
Next meeting
The next TRIPS Council meeting is scheduled for 12-13 October 2022.

Problems viewing this page? If so, please contact [email protected] giving details of the operating system and web browser you are using.
The June 17, 2022 WTO Ministerial Decision on the TRIPS Agreement
Updated Friday, 17 June 2022, 11:30 AM Geneva time.
Early Friday morning, 17 June 2022, the WTO’s 12th Ministerial Conference (chaired by Timur Suleymenov, Kazakshstan), adopted a Ministerial Decision on the TRIPS Agreement (WT/MIN(22)/W/15/Rev.2). The text can be found here .
There were several changes since the June 10 version. The agreement is a limited and disappointing outcome overall that is most accurately described as a narrow and temporary exception to an export restriction, not a waiver.
The Clarifications
There are several so called clarifications in the text on topics, which restate the existing flexibility in TRIPS, sometimes at the risk here of making the provisions seem exceptional, although some developing countries have welcomed them. Among the clarifications, none of which add new legal benefits, are paragraphs 2, 3(a) and 4 of the agreement. The Clarification offered on Article 39.3 of the TRIPS is somewhat helpful, but essentially restates the existing safeguard already part of 39.3. The clarification on paragraph 3(d) and footnote 4 on remuneration do not change TRIPS standards, but will be helpful in national settings, including by the fact that the WTO Ministerial Decision is citing “the Remuneration Guidelines for Non-Voluntary Use of a Patent on Medical Technologies published by the WHO (WHO/TCM/2005.1).” (I am the author of that document).
The TRIPS restriction on exports under Article 31
The TRIPS agreement contains 73 Articles describing various obligations on WTO members as regards the granting and enforcement of intellectual property rights.The original waiver proposal would have provided a clean waiver of 40 Articles in the TRIPS, as regards the manufacturing and supply of any COVID 19 countermeasure. The new considerably scaled back agreement focuses on just one part of the agreement, the 20 word paragraph 31.f which limits exports made under a non-voluntary authorization, often referred to as a compulsory license. The text reads.
(f) any such use shall be authorized predominantly for the supply of the domestic market of the Member authorizing such use;
Article 31.f of the TRIPS is controversial, and often considered an embarrassment to the WTO, because it is designed to limit the economies of scale for products manufactured under a compulsory license, and it also has a very differential impact on countries depending upon their size. For big economies like the U.S., China, India or to some extent Brazil, the impact is less severe than would be for a country like Chile, Ecuador, Portugal, New Zealand, or Thailand, but for every country, it is an odd provision for an organization created to liberalize trade and exploit comparative advantages.
The original TRIPS agreement contains several important workarounds from the 31.f restrictions on exports. Most obviously, Article 31.k of TRIPS waives 31.f, when a compulsory license is a remedy to an anti-competitive practice, which can include, among other grounds, a finding that prices are excessive, or that a patent holder refuses to license a technology on reasonable terms, or that the patented invention is an essential facility, all highly relevant grounds for a vaccine compulsory license.
There is also the possibility to export under Article 30 of the TRIPS, if an exception passes a three step test. The Article 30 approach was strongly supported by health NGOs, generic manufacturers, the World Health Organization (WHO), and several countries in the 2002.2003 negotiations over this issue, and even tested in a 2000 WTO TRIPS dispute case ( DS114 ) relating to the early working of patented inventions, but opposed by the European Union, which favored what is now Article 31bis of the TRIPS.
Arguably the best way to address the export issue is found in the enforcement section of the TRIPS. Under Article 44 of TRIPS, a government or a judicial authority can limit the remedies to infringement to the payment of royalties, including cases where 100 percent of manufacturing output is exported.
In terms of state practice, the Articles 31.k and 44 approaches are the most widely used, by far. (More on the export alternatives here .)
Article 31bis
Article 31bis of the TRIPS was initially adopted by the WTO General Council on August 30, 2003, as an optional waiver of Article 31.f. The core elements of 31 bis are notifications to the WTO, anti-diversion measures, restrictions on eligibility and scope.
31bis applies to drugs, vaccines and some diagnostic tests. It is permanent. It applies to all diseases. 31bis limits the members that can benefit as importers.
In the 19 years since its adoption, the 31bis mechanism has been successfully used only once, by Apotex in Canada for an export of an HIV drug to Rwanda, working with MSF. Apotex indicated it would never attempt to use the mechanism again, giving the complexity and delays they experienced. The Article 31bis mechanism is implemented through national laws, and much of the problems Apotex faced were due to Canadian law and the Canadian government administration of the statute. Few countries have bothered to implement Article 31bis in national statutes, but among those that have, the statutes in India and China offer far more streamlined approaches. India’s version is Section 92a of the patents act, titled “Compulsory licence for export of patented pharmaceutical products in certain exceptional circumstances.” The first two paragraphs in Section 92A of the India patents act read as follows:
(1) Compulsory licence shall be available for manufacture and export of patented pharmaceutical products to any country having insufficient or no manufacturing capacity in the pharmaceutical sector for the concerned product to address public health problems, provided compulsory licence has been granted by such country or such country has, by notification or otherwise, allowed importation of the patented pharmaceutical products from India. (2) The Controller shall, on receipt of an application in the prescribed manner, grant a compulsory licence solely for manufacture and export of the concerned pharmaceutical product to such country under such terms and conditions as may be specified and published by him.
To date, the India statute has not been tested, but pending compulsory licenses for COVID therapeutics in Latin America may provide timely tests, if any of the cases succeed in the Latin American county.
It is important to note that Article 31bis of the TRIPS is very long, including an Article 31bis, an “Annex to the TRIPS” and and “Appendix to the Annex to the TRIPS Agreement.” The WTO analytical index for 31bis is more than five pages single spaced.
Article 31bis notifications
The Article 31bis notifications requirements are seen as a significant problem, in part because governments have to make notifications to the WTO before the exports take place, and also that the notifications involve quantities, even in cases where the importing country is not certainly how many units to purchase, or when the government is not the sole market for the product.
Governments around the world often see the use of compulsory licensing of patented inventions as politically sensitive, inviting considerable pressure from the United States, the European Union and several of its members and Switzerland. WTO notifications on the prior use of a compulsory license will typically involve several ministers or agency heads, including those working on trade, foreign affairs, health and intellectual property rights, if not heads of state. 31bis requires such notifications from both importing and exporting countries. By, as a practical matter, involving trade and foreign affairs officials, there is a greater opportunity for bilateral pressures to block actions. KEI has worked on multiple cases where importing countries were not willing to make a notification as potential importers without having a committed supplier, and the suppliers could not get their governments to make a notification regarding exporting, without the importing country having made its notifications. All of this has to be repeated for each authorization, which includes specifications of qualities and designations. These were the Rwanda and Bolivia notifications as importers, the only two received by the WTO in 19 years. ( Bolivia , Rwanda ), and Canada as an exporter, the one time an export was actually approved under the 31bis mechanism. ( Canada )
Article 31bis anti-diversion obligations Article 31bis does contain several sections regarding the obligations on importing and exporting countries to prevent the re-exportation of the products. Governments are not sure how burdensome the obligations are in practice, but they do go beyond the obligations included in the pre-31bis TRIPS text, and seem to be conflict with Articles 6 of the TRIPS on the exhaustion of rights. They may also increase the costs to the supplier if they cannot re-export unused products when the forecast demand is not met in one country.
The new Ministerial Decision on the TRIPS Agreement
The new Ministerial Decision on the TRIPS Agreement provides an exception to 31.f export restrictions that is temporary, applies only to vaccines and only to COVID 19, limits which countries and import or export, and contains notification and anti-diversion oblations.
Eligibility for importers and exporters
As noted, Article 31bis provides no limits on which countries can use the exception as exporters, but does limit the eligible importing countries. The 31bis limits on importing eligibility is implemented through an opt-out process, and countries were allowed to opt out as importers in general (37 members have done so, see open letter of April 7, 2020 ), or to declare they would only use the mechanism in emergencies.
The new Ministerial Decision on TRIPS defines eligibility for both importing and exporting in footnote 1:
For the purpose of this Decision, all developing country Members are eligible Members. Developing country Members with existing capacity to manufacture COVID-19 vaccines are encouraged to make a binding commitment not to avail themselves of this Decision. Such binding commitments include statements made by eligible Members to the General Council, such as those made at the General Council meeting on 10 May 2022, and will be recorded by the Council for TRIPS and will be compiled and published publicly on the WTO website.
While the opt-out language on eligibility is similar to the approach taken in the August 30, 2003 waiver of Article 31.f of the TRIPS, which is now part of TRIPS as 31 bis , there is an important difference. The new agreement limits the countries eligible to import or export.
There is an expectation that all non-developing countries with the current ability to manufacture and export vaccines will opt out. The status of China seems to be addressed in the reference to the May 10 meeting of the General Council, where China expressed willingness to opt out. This is in some ways an astonishing result. The WTO will continue to enforce export restrictions on vaccines from most of the leading vaccine manufacturing countries including many with sophisticated technology. At an NGO briefing during the negotiations, WTO DG Ngozi Okonjo-Iweala seemed to justify this outcome on the grounds that it would be desirable protectionism to achieve the objective of promoting vaccine manufacturing capacity in Africa and other developing countries.
Notifications
The new Decision had marginally less problematic notifications, the most notable improvement is that notifications can be made “as soon as possible after the information is available,” and while it is not exactly clear how different the requirement is, it does seem like an improvement from 31bis.
Anti-diversion
On the anti-diversion language, the new Decision has one notable but perhaps narrow improvement over 31bis, stating that “in exceptional circumstances, an eligible Member may re-export COVID-19 vaccines to another eligible Member for humanitarian and not-for-profit purposes, as long as the eligible Member communicates in accordance with paragraph 5.” NGOs such as TWN have pointed of that the Ministerial Decisions begins by “Noting the exceptional circumstances of the COVID-19 pandemic,” so hard to say how to interpret the “in exceptional circumstances” language here.
The time period is 5 years, which severely limits its usefulness. Paragraph 5 states:
An eligible Member may apply the provisions of this Decision until 5 years from the date of this Decision. The General Council may extend such a period taking into consideration the exceptional circumstances of the COVID-19 pandemic. The General Council will review annually the operation of this Decision.
The exception to the export restrictions will only last 5 years. There are currently no developing country vaccines manufactured under a compulsory license. Moderna is operating under a compulsory license from the United States, but the US will not be an eligible exporter. For the Ministerial Decision to have any use for vaccines, a developing country would have to issue a compulsory license on a vaccine or vaccine input, obtain regulatory approval for that vaccine, and export more than 50 percent of output. Under optimistic scenarios, it could take 2 to 3 years to bring a new COVID vaccine into the market, given the increasing challenges in obtaining regulatory approval not that emergency use authorizations have already been used for multiple vaccines. And that would only give the developer, if they began work today, a few years of sales under the exception. For this reason alone, the Indian government has predicted the Ministerial Decision would not lead to any new vaccine manufacturing. ( June 14, 2022, Statement by Shri Piyush Goyal during the WTO 12th Ministerial Conference at the meeting with co-sponsors of TRIPS Waiver )
“Second, with great difficulty we got the period of 5 years. But, we all know that by the time we get an investor, get funds raised, draw plans, get equipment and set up a plant, it will probably take 2.5-3 years to do that. After that, you will start producing and within 2 years, you will have to bring down your exports to the normal compulsory license level and your capacity will remain idle.”
The Ministerial Decision text will be tied with 31 bis as one of the worst ways to allow exports under a compulsory license. Articles 31.k, 30 and 40 all will dominate. (More on the alternatives here ).
The big pharma industry can be pleased with the precedents on notifications and anti-diversion, which are important to them, as well as the exclusion of most vaccine manufacturers and the 5 year duration.
It is hard to imagine anything with fewer benefits than this, as a response to a global health emergency (other than the earlier negotiating texts for this Decision). The fact that the exception is limited to vaccines, has a five year duration and does not address WTO rules on trade secrets makes it particularly unlikely to provide expanded access to COVID 19 countermeasures.
The pressure this week was to reach consensus in order to make multilateralism look like it works, which seems to have been the main justification for producing this decision.
Silver linings While the text is not expected to impact COVID 19 vaccine equity much or at all, there are some silver linings.
1. In terms of precedents going forward, the texts of notifications and anti-diversion are both much shorter and more usable than 31bis. 2. There is no 31bis requirement to limit imports to countries with no or insufficient manufacturing capacity, a sometimes ambiguous standard oddly unconnected to economic feasibility. 3. The fact that the Decision cites “the Remuneration Guidelines for Non-Voluntary Use of a Patent on Medical Technologies published by the WHO (WHO/TCM/2005.1)” will be useful in national settings. 4. If the decision is extended to therapeutics in six months, it may be much more value, given the supply constraints on therapeutics and the much better regulatory pathway. For therapeutics, even the language on 39.3 will be useful for some countries. 5. It’s not a TRIPS waiver, it’s a useful edit of some problematic elements of 31bis. But it also turns attention back to national governments to do things, and not wait on the WTO.
James Love Twitter: @jamie_love
- Access to Knowledge
- Access to Medicine
- Competition
- Government Funded research
- Intellectual Property Rights
- Legislation
- Negotiations
- Nuclear Proliferation
- Orphan Drugs
- Presentations
- Public Goods
- Research and Development
- Site Articles
- Transparency

- WHAT THEY ARE SAYING: Doug McKalip Confirmed as USTR Chief Agricultural Negotiator
- Statement from Ambassador Katherine Tai Following Doug McKalip’s Senate Confirmation Vote
- Statement from USTR Spokesperson Adam Hodge
- USTR Announces Additional Senior Staff Members
- United States Requests New USMCA Dispute Consultations on Canadian Dairy Tariff-Rate Quota Policies
- Readout of Ambassador Jayme White’s Meeting with Mexico’s Under Secretary of Economy Alejandro Encinas
- Ambassador Tai Requests USITC Investigation of COVID-19 Diagnostics and Therapeutics
- Joint Statement from Ambassador Tai and Secretary Vilsack after Meeting with Mexican Government Officials
- USTR Extends Exclusions from China Section 301 Tariffs
- Joint USTR and Department of Commerce Readout of the First Indo-Pacific Economic Framework Negotiating Round
- Statement by Ambassador Katherine Tai on the Signing of the Memorandum of Understanding on Cooperation for Trade and Investment Between the United States and the African Continental Free Trade Area
- Readout of Ambassador Katherine Tai and Ambassador Sarah Bianchi’s Events on Day Two of the U.S. – Africa Leaders Summit
- Readout of the African Growth and Opportunity Act Ministerial During the U.S.-Africa Leaders Summit
- Readout of Ambassador Katherine Tai’s Meeting With Kenya’s Ministry of Investments, Trade and Industry, Cabinet Secretary Moses Kuria
- What They Are Saying: Ambassador Katherine Tai Confirms Amendments to the Beef Safeguard Trigger Level Under the U.S.-Japan Trade Agreement
- Protocol Amending the Beef Safeguard Provisions of the U.S.-Japan Trade Agreement to Enter into Force on January 1, 2023
- Readout of Ambassador Katherine Tai's Meeting with Canada's Minister of Labor Seamus O'Regan
- United States and Bangladesh Convene 6th Meeting of the U.S.-Bangladesh Trade and Investment Cooperation Forum Agreement Council
- Joint Statement on the Third Meeting of the United States – Argentina Council on Trade and Investment
- United States and Panama Hold the Third Meeting of the Environmental Affairs Council under the United States-Panama Trade Promotion Agreement
U.S. to Support Extension of Deadline on WTO TRIPS Ministerial Decision; Requests USITC Investigation to Provide More Data on COVID-19 Diagnostics and Therapeutics
- U.S.-EU Joint Statement of the Trade and Technology Council
- USTR, Department of Labor, European Commission Host Inaugural Principals’ Meeting of the U.S.-EU Trade and Labor Dialogue with Union, Business Leaders
- United States and European Union Conclude First Ministerial Meeting of the Large Civil Aircraft Working Group
- United States and Peru Hold Meetings of the Environmental Affairs Council and the Sub-Committee on Forest Sector Governance under the United States-Peru Trade Promotion Agreement
- Readout of Ambassador Katherine Tai's Meeting with Mexico’s Secretary of Economy Raquel Buenrostro
- PRESS ADVISORY: Media Registration for Third U.S.-EU Trade and Technology Council Ministerial Meeting
- Policy Offices
- Press Office
- Press Releases
December 06, 2022
USTR releases summary of five-month consultation on extending WTO TRIPS decision showing broad divergence of views
WASHINGTON – The Office of the United States Trade Representative today announced support for extending the deadline to decide whether there should be an extension of the World Trade Organization (WTO) Ministerial Decision on the TRIPS Agreement (Ministerial Decision) to cover the production and supply of COVID-19 diagnostics and therapeutics. USTR also announced that it will ask the United States International Trade Commission (USITC) to launch an investigation into COVID-19 diagnostics and therapeutics and provide information on market dynamics to help inform the discussion around supply and demand, price points, the relationship between testing and treating, and production and access.
“Over the past five months, USTR officials held robust and constructive consultations with Congress, government experts, a wide range of stakeholders, multilateral institutions, and WTO Members,” said Ambassador Katherine Tai. “Real questions remain on a range of issues, and the additional time, coupled with information from the USITC, will help the world make a more informed decision on whether extending the Ministerial Decision to COVID-19 therapeutics and diagnostics would result in increased access to those products. Transparency is critical and USTR will continue to consult with Congress, stakeholders, and others as we continue working to end the pandemic and support the global economic recovery.”
Supporters and opponents of extending the Ministerial Decision to COVID-19 diagnostics and therapeutics provided extensive views and arguments. USTR officials also reviewed and analyzed published information, opinions, and analysis. In both cases, the views concern both the system as a whole – whether existing WTO intellectual property protections are an impediment to access to medicines or a critical element of innovation – as well as the specific characteristics of the markets for COVID-19 diagnostics and therapeutics.
The United States respects the right of its trading partners to exercise the full range of existing flexibilities in the TRIPS Agreement, such as in Articles 30, 31, and 31 bis , and the Doha Declaration on the TRIPS Agreement and Public Health, as well as the flexibilities in the Ministerial Decision. These existing flexibilities are available as part of the effort to scale up the production and distribution necessary to overcome the challenges of the ongoing COVID-19 pandemic.
Based on available data and public input, the USITC study will explore key issues such as:
- An overview of the products, focusing on WHO-approved COVID-19 diagnostics and therapeutics, including key components, the production process, intellectual property protections, and a description of the supply chain (including the level of diversification in the supply chain);
- Information on the global manufacturing industry for these products, including information on key producing countries, major firms, and production data, if available;
- Information on the global market for COVID-19 diagnostics and therapeutics, including information on demand and, to the extent practicable, an assessment of where unmet demand exists for key products and contributing factors; market segmentation; and supply accumulation and distribution;
- Data and information on global trade in COVID-19 diagnostics and therapeutics, if available, or if not, data and information on global trade in diagnostics and therapeutics generally; and
- A brief overview/background of the relevant aspects of the TRIPS Agreement and the United Nations (UN) Medicine Patent Pool (MPP) and a listing of countries seeking to use the Ministerial Decision and those utilizing access to COVID-19 medicines under the MPP.
As part of the Administration’s comprehensive effort to combat the pandemic, the United States supported negotiations that resulted in the WTO issuing the Ministerial Decision on the TRIPS Agreement on June 17, 2022. Since then, USTR officials consulted with Members of Congress and more than two dozen stakeholders, including public health advocates, organized labor, academics, think tanks, companies, and trade associations. USTR has summarized the diverse views heard during the five-month consultation period.

- 600 17th Street NW
- Washington, DC 20508

- Reports and Publications
- Fact Sheets
- Speeches and Remarks
- Blog and Op-Eds
- The White House Plan to Beat COVID-19
- Free Trade Agreements
- Organization
- Advisory Committees
- USTR.gov/open
- Privacy & Legal
- FOIA & Privacy Act
- Attorney Jobs
- Armed Conflict
- Economic Development
- Global Health
- Human Rights
- International Criminal Court
- Inter-State Arbitration
- Book Discussion
- EJIL: Live!
- EJIL: The Podcast!
- Announcements
- Contributing a Blog Post
- Contributing an Announcement
Analysis of the 12th WTO Ministerial Conference Decision on the TRIPS Agreement
The MC12 TRIPS Decision makes available a new waiver, with respect to COVID-19 vaccines, of an existing obligation contained in TRIPS Article 31(f) that states that exports under a compulsory licence must be restricted to the non-predominant part of the authorized amount. However, paragraph 9 of the Decision explicitly states that it is without prejudice to existing flexibilities under the TRIPS Agreement, except with respect of paragraph 3(b) that sets out the new waiver. This means inter alia that neither Article 31(f), which still allows the non-predominant part of supply under a compulsory licence to be exported, nor Article 31 bis , which incorporates the first waivers given of Article 31(f), are superseded by this Ministerial Decision. Thus, these two options remain open, there being no restriction on products under Article 31 and pharmaceuticals, being the only products covered under Article 31 bis .
It is unclear why instead of further simplifying Article 31 bis , WTO Members chose to add a third option through this Decision on COVID-19 vaccines. The waiver of Article 31(f) contained in this Decision is qualified and is subject to several conditions, as is the case in Article 31 bis . The analysis below will try to throw light on how the Decision differs from existing two options from the perspective of developing country Members. This blog first sets out the features of the MC12 TRIPS Decision that are favourable, or at least neutral vis-à-vis the existing options, and then details those that are less favourable or even more restrictive than the existing options. No claim is made to comprehensiveness.
Features of the MC 12 TRIPS Decision that are favourable or neutral vis-à-vis existing options for compulsory licensing under TRIPS
Alternative instruments to grant use without authorization : It has been clarified in paragraph 2 of the Decision that the “law of a Member” referred to in the chapeau of Article 31 is not limited to legal provisions on compulsory licensing, but also includes other acts, such as executive orders, emergency decrees, and judicial or administrative orders. While use of such alternative instruments may not have been ruled out in Article 31 nor Article 31 bis , this appears to have been a useful clarification for some developing country Members.
No requirement of prior authorisation of right owner : Unlike in Article 31 bis, developing country Members that use this Decision need not require the proposed user in their jurisdictions to make efforts to obtain a voluntary licence under reasonable commercial terms within a reasonable period of time as set out in Article 31(b). In practice, Members implementing Article 31bis did have the flexibility to incorporate very short periods of time to try to obtain such licences.
Notifications required but different: The notification requirements to the WTO for developing country Members that use this Decision, either as importers or as exporters, are set out in its paragraph 5 and the corresponding footnote. Unlike in Article 31 bis , both sets of Members will have to notify the name and address of the authorized entity, the products for which the authorization has been granted and the duration of the authorization. In addition, the quantities for which the authorization has been granted and the countries to which the products are to be supplied must be notified (presumably by the exporting Member) as soon as possible after the information is available. However, unlike in Article 31 bis , importing country Members do not have to notify their intention to be importing Members, nor self-declare (if they are not least developed country Members) that they do not have manufacturing capacity to make these vaccines themselves.
No distinctive marking for exported COVID-19 vaccines: An important difference with Article 31 bis is that exporting developing country Members do not have to require exporters to specifically label or mark their products to distinguish them from originator products. Perhaps, this requirement was removed since there is now an absolute requirement on importing Members to prevent re-exportation (see below). Consequently, exporting country Members do not have to notify the WTO of the distinguishing features of their labelling and marking of COVID-19 vaccines. Since all manufacturers do have to mark and label their exports, the advantage gained may be debatable.
Features of the MC12 TRIPS Decision that are less favourable vis-à-vis existing options to export under a compulsory licence under TRIPS
Use limited to developing country Members : Unlike in Article 31 and Article 31 bis , only developing country Members of the WTO are eligible to use the Decision either as an importing or as an exporting Member. Indeed, developing country Members with existing capacity are encouraged in footnote 1 of the Decision to make a binding commitment not to avail themselves of the Decision. China’s statement in the General Council meeting on 10 May 2022 is taken as one such binding commitment. The reasoning behind this restriction – whether realistic or not – appears to be that since the goal of the proponents of the original waiver proposal (in IP/C/W/669 and its revision) was to encourage local manufacture in developing countries, the Decision should be used more by those that do not currently have manufacturing and export capacity for COVID-19 vaccines. The goal under Article 31 bis differed – it was to supply generic medicines under export compulsory licences to Member countries that lacked manufacturing capacity, irrespective of where these were manufactured.
Stricter obligation on re-exportation : Those developing country Members that do import under this Decision have a binding obligation to undertake all reasonable efforts to prevent the re-exportation of the products manufactured under this Decision. The Decision allows in footnote 3 that, “in exceptional circumstances”, such an importing Member may re-export COVID-19 vaccines to another developing country Member for humanitarian and not-for-profit purposes, as long as such transactions are notified to the TRIPS Council. In contrast, in the terms set out in in paragraph 3 of the Annex to the TRIPS Agreement, this same obligation was heavily negotiated and is qualified. Here importing Members are only obliged to take measures to prevent re-exportation when such measures are 1) reasonable, 2) within their means, 3) proportionate to their administrative capacities, and 4) proportionate to the risk of trade diversion.
No double remuneration exemption: Compulsory licences – whether issued in the exporting or importing country Members – are subject to adequate remuneration under Article 31(h). However, unlike paragraph 2 of Article 31 bis , which exempts the importing Member from paying remuneration once it has already been paid in the exporting country for the same products and quantities, this Decision makes no mention of this “no double remuneration” clause. This means that those importing developing country Members that use this Decision to import COVID-19 vaccines under a compulsory licence are not explicitly exempted under it from paying adequate remuneration, unlike under Article 31 bis . Since importing Members have to change domestic laws to be exempt from paying patent owners, legal certainty on this point is important.
New standard for remuneration: Deviating from language used in Article 31 bis where remuneration has to be based on the economic value of the authorisation to the importing Member, this Decision makes it optional to take into the account the humanitarian and not-for-profit purpose of the specific vaccine distribution programs aimed at “providing equitable access to COVID-19 vaccines in order to support manufacturers in eligible Members to produce and supply these vaccines at affordable prices for eligible Members.” (Emphasis added). Thus, a new standard of requiring a humanitarian, not-for-profit royalty rate, which results in both supporting local production in exporting developing countries as well as affordable prices in importing developing countries, is set in this Decision. It is unclear why footnote 4 was added since the paper referred to therein does not explain how such a balance could be found.
Ambiguity on test and other data protection : Paragraph 4 of the Decision states – as if it is a fact – that it is understood that TRIPS Article 39.3 does not prevent a developing country Member from enabling rapid approval for use of a Covid-19 vaccine produced under this Decision. Article 39.3 in TRIPS itself does not mandate regulatory data exclusivity and allows such data to be disclosed in public interest. Article 31 and Article 31 bis are silent about the relationship between the test data and compulsory licence provisions, giving Members the freedom to suspend any data exclusivity provisions in place to benefit the compulsory licensee. In any case, those Members that have introduced regulatory data exclusivity, pursuant to bilateral/plurilateral Free Trade Agreements, may have less flexibility unless those commitments are amended separately.
No special waiver for Regional Trade Agreements with majority LDC membership: Unlike the provision in paragraph 3 of Article 31 bis that was aimed at benefiting African countries in that they need not notify the WTO of use if it was within a RTA with majority membership of least developed countries, there is no corresponding provision in this Decision. This second waiver of Article 31(f) has never been used but was seen as valuable in those negotiations.
Limited duration of the Decision : Unlike in Article 31 or Article 31 bis where there are no time limits, developing country Members can issue authorisations under this Decision for a period of five years from the date of adoption of this Decision. This period could be extended by the General Council taking into account the “exceptional circumstances” of COVID-19 at that time. Presumably, a limited waiver was warranted for a time-limited pandemic. Even the proponents of the original waiver sought the duration of three years in IP/C/W/669/Rev.1, albeit for a much broader waiver of several TRIPS provisions. However, it is unclear whether the authorisations already issued by this date to private companies/third parties could continue to be valid beyond this date as there appears to be no time limit laid down for such authorisations, other than the logical limit up to patent expiry, just as in the case of Article 31 (except a generic requirement to rescind these once the purpose is met) or in Article 31 bis .
To conclude, this Decision includes provisions that, on the one hand, facilitate use without the authorisation of the right holder for the export/import of COVID-19 vaccines and, on the other hand, either includes those that are stricter or cause more legal uncertainty than the existing provisions, or ignores several existing provisions in Article 31 bis that are favourable for developing countries. Thus, it is legitimate to question whether, on balance, this Decision makes the terms and conditions of waiving Article 31(f) more or less favourable for developing country Members for the export and import of COVID-19 vaccines than those already available inter alia under Article 31(f) and 31 bis . Since these existing legal avenues for export under a compulsory licence remain open, it remains to be seen if the Decision will be acted upon by developing country Members. However, the clarification of points of legal uncertainty may need to be borne in mind when WTO negotiations, which are to be concluded within six months, begin on the inclusion of COVID-19-related diagnostics and therapeutics.

Share this:
- EJIL Analysis
No tags available
Leave a Comment
Comments for this post are closed
Jerome Reichman says
July 9, 2022
Very helpful . Well done, best wishes, Jerry Reichman
Jayashree Watal says
July 12, 2022
Thanks Jerry - am glad you found it helpful. Warmly, Jayashree
Fernando dos Santos says
July 14, 2022
Many thanks for this analysis Prof. I am indeed trying to write something on the added value of this Decision. This gave me some insights. Fernando dos Santos
- TRIPS Flexibilities Database
- Pandemic Accord
- Our research
- Introduction
- Model Government Use Licences
- Model Uses of Paragraph 7 or “LDC Waiver”
- Special Compulsory Licences for Export of Medicines
- Options for Regional Economic Communities
- Voluntary Licences
- Useful Links
Explore the website
Technical briefing papers.
Deep dives into and analysis of issues that impact access to medicines.
Our Work on Covid-19
Our views on what governments and the international community can do to fight this pandemic and prepare for the next one.
Timely and topical analysis on the day-to-day issues in intellectual property and health.
The TRIPS Flexibilities Database
Instances when authorities have invoked, planned to/were asked to invoke a flexibility contained in the TRIPS Agreement for public health reasons.
Tools for Access to Medicines
Tools to help governments and others looking to increase access to medicines by using flexibilities in intellectual property law.
Meet the Team
Learn about Medicines Law & Policy's team of legal and policy experts.
Subscribe to our newsletter and never miss a post!
Medicines Law & Policy will use the information you provide to keep you up-to-date when we post new research and insight. You can change your mind about receiving our newsletter any time by clicking the unsubscribe link in the footer of any email you receive from us. We will treat your information with respect. By clicking below, you agree that we may process your information in accordance with these terms.
We use Mailchimp as our marketing platform. By clicking below to subscribe, you acknowledge that your information will be transferred to Mailchimp for processing. Learn more about Mailchimp's privacy practices here.
New reports offer evidence for extending the WTO Decision on TRIPS to Covid-19 therapeutics and diagnostics. Why delay further?

In February 2024, the 13th World Trade Organization (WTO) Ministerial Conference will take place. WTO Members are expected to decide whether to extend the June 17 2022 Ministerial Decision on the TRIPS Agreement, which is currently aimed at increasing access to Covid-19 vaccines, to therapeutics and diagnostics. The original deadline for this decision was December of 2022.
The June 17 Ministerial Decision reinforces countries’ rights to use flexibilities contained in the WTO’s Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement. It also lifts, for Covid-19 vaccines, the export restrictions contained in TRIPS Article 31 (f) which determines that products produced under a compulsory licence must be predominantly for the domestic market. It further clarifies that a Member may authorise the use of the subject matter of a patent without the consent of the patent holder by any means available, whether the Member has rules for compulsory licensing (CL) or not. This is particularly useful for countries without procedures or with cumbersome procedures for issuing a CL. The decision also stipulates that TRIPS Article 39.3 (which protects against ‘unfair commercial use’ of test data needed to register a medicine) does not prevent the rapid marketing authorisation by medicines regulatory bodies. The Decision is currently limited to Covid-19 vaccines but would be more useful for other products, in particular therapeutics. According to the World Intellectual Property Organization (WIPO ), 4,787 patent applications for Covid-19 therapeutics were filed between January 2020 and the end of September 2022. Further, it may be easier for effective use of the Decision to be applied to therapeutics and diagnostics as these can often be produced without access to additional know-how or technology.
In recent weeks, two documents have been published that should help to nudge Members towards extending the June 17 decision to therapeutics and diagnostics.
The first document is the long-awaited study carried out by the US International Trade Commission (USITC) upon request of the US Trade Representative Katherine Tai: COVID-19 Diagnostics and Therapeutics: Supply, Demand, and TRIPS Agreement Flexibilities (Inv. No. 332-596, USITC Publication 5469, October 2023) . The second document is the TRIPS Council Chair’s report of the TRIPS Council’s Informal Thematic Session for External Stakeholders held at the WTO on 28 September 2023.
Neither report offers firm “yes or no” recommendations, but the data and evidence presented in both make a compelling case for extending the Decision to therapeutics and diagnostics.
The 493-page USITC report contains reports of discussions with various stakeholders and a comprehensive literature review. The report describes the disparity in access and availability of Covid-19 diagnostics and therapeutics: 80% of government procurement was by high-income countries, 14% by upper-middle-income countries, 5% by lower-middle-income countries and none by low-income countries. The report mentions that many factors affect access but highlights that high prices and the lack of price transparency appear detrimental to many countries seeking access.
From the literature review the USITC carried out it highlights that:
“From the available evidence, patent protection is generally found to be more beneficial to innovation in the health sector for developed countries and less so for developing countries. Patent protection is often found to result in higher prices for medicines, which decrease access, but patent protection can also have some counteracting effects, such as increases in international trade flows of pharmaceuticals and faster drug launches in markets, that help improve access. Researchers have found that compulsory licenses and the MPP are associated with increased generics and lower prices, and increased access to pharmaceuticals. Researchers have not studied the relationship between compulsory licenses and the MPP and access to COVID-19 diagnostics and therapeutics.”
The USITC encourages more research into the relationship between compulsory licences and the Medicines Patent Pool (MPP)and access to Covid-19 diagnostics and therapeutics.
The report goes on to acknowledge the advantages of CLs in terms of lowering prices and increasing access to needed products. The fact that a CL does not cover know-how, trade secrets and other forms of IP may be a barrier to effectively using the CL. This is especially true for new, complex technologies such as the mRNA Covid-19 vaccines. However, less complex molecules – and several Covid-19 therapeutics fall in that category – can be readily manufactured without access to additional knowledge. Another challenge listed is the lack of generic production capacity. Many countries that have used a CL in the past have relied on imports. Therefore, the waiver of TRIPS Article 31(f) export restrictions contained in the Ministerial Decision would be most welcome for use in therapeutics.
It also points out that in the past, countries that have used a CL have come under political and trade pressure from high-income countries such as the US and EU and the pharmaceutical industry. It also expresses the expectation that the 2022 Ministerial Decision can play a role in addressing this type of pressure. It says:
“The implementation of the 2022 Ministerial Decision has been highlighted as a potential means of reducing both this political pressure and potentially limiting retaliation from the pharmaceutical sector, as it reaffirms the right to issue a CL in a similar way to the Doha Declaration on the TRIPS Agreement and Public Health .”
Using, among other sources, ML&P’s TRIPS Flexibilities Database , the report does a good job of presenting the experience with CLs since the adoption of the Doha Declaration in 2001. See the illustration below.
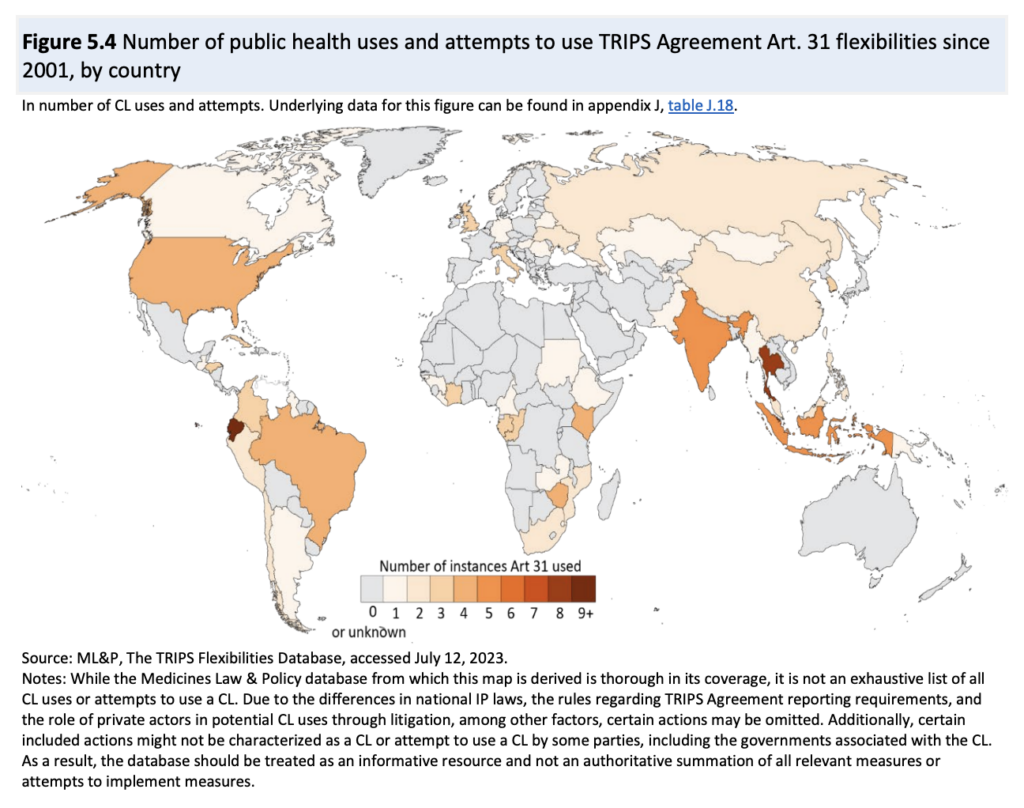
The report offers a comprehensive description of bilateral voluntary licences and Medicines Patent Pool licences. In both types of licences, the patent holder determines in which territories the products may be made available; in practice, this means that a significant number of middle-income countries are excluded from the agreements. Those excluded countries can, however, use compulsory licensing to gain access to the licensed products. MPP licences generally allow their sublicensees to supply to countries that have issued a compulsory licence. For details, see also the WHO/UNITAID country briefing . CL, therefore, remains an important option because tiered pricing by the originator does not offer the same advantages. The report says:
“In the case of COVID-19 therapeutics, tiered prices reportedly were multiples higher than the [generic] price negotiated by the Clinton Health Access Initiative.”
The USITC offers insights on the benefits of compulsory licensing, in particular for therapeutics that do not require know-how transfer. While the USITC carefully avoids taking a position on whether the June 17 2022 Decision should be extended to therapeutics and diagnostics, the evidence presented leads to the conclusion that extending it is sensible. This is how the USITC highlights the issue:
“In the available literature on the impact of CLs on pharmaceutical products, researchers have generally found that CLs are associated with decreased pharmaceutical prices in the countries that used CLs. The available research also associates CLs with increases in the number of people with access to patented products. There is some evidence that CLs encouraged innovation, where the literature has generally focused on the broader chemical industry.”
This conclusion that CLs are associated with lower drug pricing and increased access is supported by much of the evidence presented at the TRIPS Council’s Informal Thematic Session for External Stakeholders held at the WTO on 28 September 2023, and contained in the Chair’s report mentioned above.
A consensus emerged amongst most of the 22 speakers that licensing of IP is an important feature of the IP system to help enhance access to medical products. In particular, those medical products for which access to patents is sufficient to allow generic production. More complex technologies may require the transfer of additional know-how or technology.
The industry representatives stressed the role of IP in justifying R&D investments and urged the Council not to extend the decision to therapeutics and diagnostics. Offering the familiar talking point that doing so might undermine companies’ ability to invest in innovation.
This position is not supported by evidence as the USITC points out there is even some evidence that the use of CL stimulates innovation. Even in disease areas where the use of TRIPS flexibilities has been extensive, there has not been a negative effect on pharmaceutical innovation. This is not surprising because territories where TRIPS flexibilities are used, generally represent markets where the industry is absent with the products in question.
In conclusion, the two reports offer amble argumentation in favour of extending the June 17 Decision to therapeutics and diagnostics. It would, therefore, only be sensible for the WTO Members to do so. There is no reason for further delay.
- Ministerial Decision
- TRIPS Council
- TRIPS flexibilities
- US International Trade Commission

Never miss a post! Sign up for ML&P's newsletter.
Recent Articles
World trade organization members embark on review of the trips agreement, msf’s access campaign is an invaluable actor in global health; shutting it down is short-sighted., the new ip strategy of war-torn ukraine should prioritise public health and national security, pharmaceutical accountability foundation meets abbvie in court, looking to advance its pricing case, worldwide licensing of pandemic technologies is already current practice. the pandemic accord should protect it., related articles, continuing to ignore the problem of the know-how gap won’t make it go away., pressure from european countries related to the use of trips flexibilities , articles by topic, medicines law & policy.
Medicines Law & Policy releases analysis, policy recommendations and tools to aid practitioners working on universal access to medicines. We strive to provide information that is as accurate as possible, and we welcome and encourage feedback. For comments or questions contact us at: [email protected] .
- Other resources
This work is licensed under Creative Commons . You are free to share, with attribution and without changes, for non-commercial purposes.
Position Statement on the Decision of the WTO Ministerial Conference on the TRIPS Agreement
Research news.
On 17 June 2022, the WTO Ministerial Conference adopted a long-awaited decision on the TRIPS Agreement. The Decision has not waived any intellectual property rights as such, as proposed by India and South Africa in October 2020. Instead, it mainly clarifies the application of the existing TRIPS flexibilities. As a follow-up to its earlier Position Statement, the Institute issued a paper that outlines the legal and practical implications of the Decision.

This second Position Statement, which follows the Institute's Position Statement of 7 May 2021 , reflects on the legal and practical implications of the Ministerial Decision in view of the ultimate goal of overcoming the COVID-19 pandemic. A particular focus here is on TRIPS flexibilities relating to compulsory licensing of patents.
Position Statement of 5 July 2022 on the Decision of the WTO Ministerial Conference on the TRIPS Agreement adopted on 17 June 2022

11 bodies recovered in Nepal after landslide sent 2 buses into river

World's rarest whale washes up on New Zealand beach

China, Russia start naval drills days after NATO's broadside to China

Academic Freeze: Universities, colleges in Bangladesh closed indefinitely after six killed
Wto 13th ministerial conference approves extension to trips waiver.

ABU DHABI, 1st March, 2024 (WAM) -- The WTO 13th Ministerial Conference today approved an extension to the TRIPS Waiver, enabling developing countries to increase vaccine production capacity for COVID-19 and future pandemics.
The decision was one of the key objectives of the 13th WTO Ministerial Conference. Intellectual property laws account for 90 percent of non-tariff barriers to trade, so the decision will benefit least developed countries significantly.
The Ministerial Conference in its final statement also included new rules on local regulation of services, which will reduce trade costs by billions of dollars worldwide.
The WTO Ministerial Conference statement also affirmed the continuation of efforts to reform the Dispute Settlement System (DSS). This reform aims to establish a comprehensive, effective, and accessible system for all members by the end of 2024. To achieve this, the statement emphasises the need for inclusive and transparent discussions to build upon existing progress and address remaining challenges, including those related to the Appellate Body.
Additionally, WTC member countries agreed to the extension of a moratorium on e-commerce tariffs until the 14th WTO Ministerial Conference in 2026. This decision marks a significant achievement for the conference, which tackled extensive negotiations on several key issues impacting the future of global trade.
WAM 2nd March 2024, 08:21 GMT+11
Read This Next
Big News Network
- Big News Network News Agency
- Midwest Radio Network
- Mainstream Media
BIG NEWS NETWORK.COM
- Contact & Support
- Terms & Conditions
PRODUCTS & SERVICES
- News Releases
Copyright © 1998-2024 Big News Network All rights reserved. ISSN : 2616-6917
- Overview initiatives
- About this website
TRIPS Waiver
The Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) sets out minimum standards all member states of the World Trade Organization (WTO) must provide to protect intellectual property rights on novel innovations. In order to enhance global, equal access to medical products against Covid-19, in October 2020, the governments of India and South Africa proposed a so-called TRIPS Waiver.
The objective of this original TRIPS Waiver proposal was to ensure that certain obligations of the member states to protect intellectual property on Covid-19 related innovations, would be temporarily removed upon adoption by the WTO. Through this ceding of important intellectual property barriers, pharmaceutical companies that do not hold the patent rights can be allowed to use, produce and sell Covid-19 related innovations too – without fear of legal repercussions.
The proposal provoked both support and opposition. That it was also received with considerable acclaim is evident, as 65 WTO member states were co-sponsors while 105 other members expressed support. The opposition mainly came from the pharmaceutical industry and higher-income countries, where access has been significantly less of a problem due to their financial power and the residence of pharmaceutical companies within their territories.
The conflicting views resulted in a long time of discussions and negotiations. Finally, on 17 June 2022, during the 12th WTO Ministerial Conference (MC12), the Ministerial decision on the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement, aka the TRIPS Waiver, was a fact.
The decision entails a strongly watered-down version of the original TRIPS Waiver drafted by India and South Africa. It only covers vaccines and no other Covid-19 related medical products. This will be up for reconsideration within six months after the decision. Instead of the originally proposed waiving of 35 TRIPS provisions, the decision only waves one provision, Article 31 (f), allowing the export of vaccines under a compulsory license. Lastly, the duration of the decision is limited to five years.

- Has resparked the discussion on what level of intellectual property protection is desirable for medical products in health crises.
- The discussions at WTO level can be considered as a recognition of inadequacy of TRIPS in pursuing affordable access for all.
- WTO decision could accelerate up-scaling of global production capacity of Covid-19 vaccines in countries experiencing intellectual property barriers and (plan to) produce vaccines for domestic use and export most of them.
- WTO decision does not stimulate structural change.
- WTO decision does not address transfer of know-how and technology needed to safely and effectively scale-up production in the fastest way possible.
- WTO decision is limited to vaccines and thus does not include other key medical products, such as treatments and diagnostics.
- WTO decision is not a proper recognition of intellectual property rights as a barrier for increased production capacity of key medical products.
- WTO decision can be considered as a prioritisation of economical interests of high-income countries over global health equity.
- There is a risk that the decision will function as blueprint for pandemic accord negotiations, compromising meaningful provisions to achieve equitable access to medical products in future frameworks.
- Free Trade Agreements some countries have concluded, may hinder the full impact of the WTO decision.
The entire TRIPS Waiver negotiation process was a manifestation of shared dissatisfaction with TRIPS. As such, the negotiations provided fertile ground to rethink the agreement in light of access to health innovations.
However, the result of the negotiations is nowhere near the objectives determined by India and South Africa. The WTO decision barely waives provisions and merely repeats or clarifies existing options for overriding patents by compulsory licensing. It neglects essential aspects for improved access to medical products, has a limited duration (five years) and only covers vaccines and no other key medical products.
Another important aspect to note is that solely addressing patents is not enough. To be able to manufacture and market (for instance) vaccines, with assured quality built in, it is a neccessity to acquire much more information than included in patents. Information on processes, machineries, quality contol standards, etc. is vital to assure products that meet safety and effectivity requirements and have them marketed timely. This requires transfer of technology and know-how, but this is rather difficult to enforce (through, for example, compulsory licensing or waiving TRIPS provisions) and is likely to be more effective on a voluntary basis.
The WTO decision shows that commercial interests of high-income countries have prevailed over the objective to support equitable access to Covid-19 innovations. The initial proposal by India and South-Africa aimed at improving the power imbalance between governments and pharmaceutical companies, but also between high-income countries and low- and middle-income countries. The WTO decision does not contribute in any substantial way to either goal.
Nonetheless, some national governments are expected to may benefit from the WTO decision. Among them are those that are confronted with existing or potential intellectual property barriers, which are (planning on) producing vaccines for domestic use and export the majority of them. It would be highly beneficial to expand the scope of the decision to diagnostics and therapeutics too.
Text of the WTO decision (June 2022)
Devex article ‘WTO finally agrees on a TRIPS deal. But not everyone is happy’
Text of the first proposal for the TRIPS Waiver (October 2020)
Text of the revised proposal for the TRIPS Waiver (May 2021)
Content Search
Covid-19: wto ministerial decision on trips agreement fails to set rules that could save lives.
Responding to today’s ministerial decision by the World Trade Organization (WTO) on the TRIPs Agreement, Tamaryn Nelson, Amnesty International’s Researcher on Economic, Social and Cultural Rights, said:
“More than two years into the Covid-19 pandemic and the WTO still hasn’t made the changes needed to ensure everyone has access to life-saving health products when they most need them. Under the terms of this decision, hundreds of millions of people in developing countries will likely continue to be denied access to many of these products.
“This decision is unlikely to make a significant difference in global access to Covid-19 vaccines right now. And the fact that the WTO decided to postpone by six months the decision around extending the agreement to cover diagnostics and therapeutics – at this stage of the pandemic – demonstrates how the WTO is out of step with reality.
“This decision is not only a hollow response to Covid-19, but it sends the message that intellectual property rights outweigh the rights to health and life. After more than 18 months of discussion, the WTO has missed an opportunity to use its power to set global trade rules that save lives, setting a worrying precedent for international cooperation in future public health emergencies.”
The WTO’s Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement sets out minimum standards for many forms of intellectual property (IP), such as copyrights, trademarks, patents, undisclosed information (including trade secrets and test data) and anti-competitive practices.
As IP rights can create barriers to timely access to lifesaving health products the TRIPS Agreement includes safeguards known as “flexibilities” so states can amend their laws and take certain measures to address public health emergencies, such as issuing compulsory licenses that would allow a company to produce a lifesaving drug without following IP rules.
The Covid-19 pandemic has raised questions about whether the “flexibilities” are effective to address the world’s urgent needs, given that they usually apply on a country-by-country, case-by-case, and drug-by-drug basis and have onerous reporting requirements.
In October 2020, India and South Africa requested a temporary waiver (IP/C/W/669) to intellectual property protections that would allow countries to produce versions of Covid-19 products more easily. Despite receiving support from more than 100 countries, this draft has stalled due to opposition from a small number of wealthy states.
A new draft ministerial decision spearheaded by the WTO Director General (WT/MIN(22)/W/15) but largely based on proposals from the European Union, was discussed and eventually adopted at the WTO’s 12th Ministerial Conference (MC12) held from 12 to 17 June 2022. Rather than waive intellectual property protections, it provides some clarifications to current “flexibilities” and a narrow exception to an export restriction on Covid-19 vaccines for the duration of five years.
Related Content
World + 6 more
Alertes de maladies épidémiques et émergentes en Océanie le 16 juillet 2024
World + 41 more
Polio this week as of 17 July 2024
World + 2 more
Not Invisible: Strengthening Protection for Children with Disabilities in Migration and Displacement
Health impacts of climate change: evidence landscape and role of private sector: insight report (june 2024).
- 2020 Global Congress
- 2018 Global Congress
- 2015 Global Congress
- Conference website, hosted by Open AIR
- Survey of Global Congress Community Research Projects
- 2012 Global Congress
- 2011 Global Congress
- IP & Covid-19
- User Rights Network
Select Page
The COVID-19 TRIPS Waiver and the WTO Ministerial Decision
Posted by Peter Yu | Jul 7, 2022 | Academic Resources , Coronavirus , Multilateral Fora
Author: Peter Yu
Abstract: In October 2020, India and South Africa submitted an unprecedented proposal to the WTO, calling for the partial suspension of the TRIPS Agreement to facilitate the “prevention, containment or treatment of COVID-19.” Although this proposal immediately received considerable support from other WTO members, civil society organizations and individual experts, it faced strong opposition from some developed countries—most notably the European Union, the United Kingdom, Switzerland and, to some extent, also the United States.
By December 2021, it was quite clear that the COVID-19 TRIPS waiver proposal would not receive enough support to achieve consensus within the WTO membership. Around that time, the European Union, India, South Africa and the United States, with the support of the WTO, launched quadrilateral consultations to find a compromise solution. The “Quad proposal” that was eventually developed through these high-level consultations became the blueprint from which WTO members developed a new ministerial decision at the Twelfth WTO Ministerial Conference in Geneva in June 2022. This decision allowed WTO members to manufacture COVID-19 vaccines—and, if subsequently approved, also other COVID-19 health products—without the authorization of the relevant patent holders.
This chapter traces the TRIPS waiver debate from the submission of the original proposal by India and South Africa in October 2020 to the final adoption of the Ministerial Decision on the TRIPS Agreement in June 2022. The chapter further evaluates the strengths and weaknesses of this newly adopted decision, comparing it with the earlier TRIPS waiver proposal. It concludes by offering suggestions for future actions that WTO members on both sides of the waiver debate could take to help combat the COVID-19 pandemic.
Citation: Yu, Peter K., The COVID-19 TRIPS Waiver and the WTO Ministerial Decision (June 30, 2022). IPR IN TIMES OF CRISIS: LESSONS LEARNED FROM THE COVID-19 PANDEMIC, Jens Schovsbo, ed., Edward Elgar Publishing, 2023, Forthcoming, Available at SSRN: https://ssrn.com/abstract=4150090 or http://dx.doi.org/10.2139/ssrn.4150090
Related Posts
Updated wipo study on copyright limitations and exceptions for libraries and archives.
November 19, 2014
Copyright and Economic Viability: Evidence from the Music Industry
December 18, 2020

Cracking the Copyright Dilemma in Software Preservation: Protecting Digital Culture Through Fair Use Consensus
November 18, 2019
WIPO Adopts Open Access Policy for its Publications
November 16, 2016
infojustice.org is hosted by the Program on Information Justice and Intellectual Property at American University Washington College of Law.
Infojustice Roundup
Archives | Subscribe
Free to Share

Blog Categories
Coronavirus Trade Agreements NAFTA TPP ACTA Domestic Legislation Domestic Policy & Litigation Limitations and Exceptions Creative Commons Multilateral (WIPO, WTO, UN) Takedowns Access to Medicines Empirical Research
Ahmed Abdel Latif Alek Tarkowski Alexander Dent Allan Rocha de Souza Andres Guadamuz Andres Izquierdo Brandon Butler Brook Baker Burcu Kilic Carolina Botero Carolina Rossini Caroline Ncube Carrie Sager Celeste Drake Christophe Geiger David Levine Eve Gray Hafiz Aziz ur Rehman Heesob Nam Hong Xue Jonathan Band Jorge Contreras Jimmy Koo Joana Varon Joe Karaganis Krista Maier Lina Diaz Leanne O’Donnell Marcela Palacio Puerta Matthew Rimmer Matthew Webb Michael Carroll Michael Geist Michael Palmedo Michael Smith Miguel Morachimo Meredith Jacob Margot Kaminski Pedro Mizukami Peter Maybarduk Pranesh Prakash Rashmi Rangnath Sangeeta Shashikant Sara Bannerman Stela Bivol Sean Flynn Stephanie Rosenberg Susan Chalmers Thiru Balasubramaniam William Xu Peter Yu
Comments on:
- An error has occurred, which probably means the feed is down. Try again later.
Civil Society Documents
Industry | infojustice.
- Aspen Doesn’t Want You to Own Your Own Casebooks
- Still Profitable After All These Years
- Recording Industry Reports Growth from User-Generated Content and New Projects in Emerging Economies
- EC Trade Negotiators and Industry Discuss How to ‘Re-Educate’ Public About Intellectual Property Rights (talk about employment)
- Witnesses at U.S. Trade Hearing Offer Opinions (and Warnings) on Intellectual Property in Upcoming Negotiations with the EU

- GET STARTED
- JOIN A GROUP
- JOIN BOOK CLUB
- WRITE FOR RIGHTS
- URGENT ACTION NETWORK
- YOUTH PROGRAMS
- UPCOMING EVENTS
- WAYS TO GIVE
- MONTHLY DONATIONS
- LEGACY GIVING
- IN MEMORIAM GIFT

- Amnesty Book Club
- Respect Indigenous Rights on Wet'suwet'en Territory
- Online Gender-Based Violence
- Immigration Detention
- Safe Third Country Agreement
- End Fossil Fuel Subsidies
- No More Stolen Sisters
- WRITE FOR RIGHTS | Write a letter. Save a Life.
- GAZA CRISIS | Activism Toolkit
- ISRAEL | Stop All Arms Sales to Israel
- UKRAINE | Demand Justice for Ukraine
- RUSSIA | War Censorship Laws Must Go
- PERU | Speak Up to Support Families Seeking Justice
- DRC | End Forced Evictions
- Amnesty Canada's Annual Human Rights Agenda
- Amnesty International's Global Annual Human Rights Report
- The Candle Magazine
- Member Publications
- Amnesty International Death Penalty Report
- Criminalization, Intimidation and Harassment of Wet'suwet'en Land Defenders
- "On my campus, I am afraid”: China’s targeting of Canadian students stifles rights
- Amnesty International Canada ‘alarmed’ by police raids on campus protest encampments
- Human rights organizations sound alarm as Canada-Ecuador trade talks get underway
- Amnesty International Canada lays out human rights priorities ahead of next federal election
- Amnesty International Canada condemns ‘appalling’ anti-trans policy changes in Alberta
- Amnesty International Canada to make arguments in court in Black Class Action case
- US veto of ceasefire resolution displays callous disregard for civilian suffering

Covid-19: WTO ministerial decision on TRIPS Agreement fails to set rules that could save lives
Responding to today’s ministerial decision by the World Trade Organization (WTO) on the TRIPs Agreement, Tamaryn Nelson, Amnesty International’s Researcher on Economic, Social and Cultural Rights, said:
“More than two years into the Covid-19 pandemic and the WTO still hasn’t made the changes needed to ensure everyone has access to life-saving health products when they most need them. Under the terms of this decision, hundreds of millions of people in developing countries will likely continue to be denied access to many of these products.
“This decision is unlikely to make a significant difference in global access to Covid-19 vaccines right now. And the fact that the WTO decided to postpone by six months the decision around extending the agreement to cover diagnostics and therapeutics – at this stage of the pandemic – demonstrates how the WTO is out of step with reality.
“This decision is not only a hollow response to Covid-19, but it sends the message that intellectual property rights outweigh the rights to health and life. After more than 18 months of discussion, the WTO has missed an opportunity to use its power to set global trade rules that save lives, setting a worrying precedent for international cooperation in future public health emergencies.”
Background
The WTO’s Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement sets out minimum standards for many forms of intellectual property (IP), such as copyrights, trademarks, patents, undisclosed information (including trade secrets and test data) and anti-competitive practices.
As IP rights can create barriers to timely access to lifesaving health products the TRIPS Agreement includes safeguards known as “flexibilities” so states can amend their laws and take certain measures to address public health emergencies, such as issuing compulsory licenses that would allow a company to produce a lifesaving drug without following IP rules.
The Covid-19 pandemic has raised questions about whether the “flexibilities” are effective to address the world’s urgent needs, given that they usually apply on a country-by-country, case-by-case, and drug-by-drug basis and have onerous reporting requirements.
In October 2020, India and South Africa requested a temporary waiver (IP/C/W/669) to intellectual property protections that would allow countries to produce versions of Covid-19 products more easily. Despite receiving support from more than 100 countries, this draft has stalled due to opposition from a small number of wealthy states.
A new draft ministerial decision spearheaded by the WTO Director General (WT/MIN(22)/W/15) but largely based on proposals from the European Union, was discussed and eventually adopted at the WTO’s 12th Ministerial Conference (MC12) held from 12 to 17 June 2022. Rather than waive intellectual property protections, it provides some clarifications to current “flexibilities” and a narrow exception to an export restriction on Covid-19 vaccines for the duration of five years.
- Human Rights News
- Press Releases
Sign up to take action
Join our movement.

- Privacy Overview
- Strictly Necessary Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.

Who are we?
The Model WTO is the biggest simulation of the World Trade Organization negotiations in the World. Our international conference provides a unique opportunity for future decision makers to experience first hand the technicalities of the multilateral trading system. Every year since 1997, students from around the world and different educational backgrounds have been invited to Switzerland to debate and exchange on the most relevant topics related to international Trade. The 27th Model WTO edition 2024 will take place form 20th to 27th of march in St.Gallen and Geneva.

Who are you?

HSG Student

Participant
- Friday, 19th July, 2024
- Rest of the World
- Health & Wellbeing
- Life & Style
At Last, FG, Labour End Weeks of Feud, Settle for N70,000 National Minimum Wage 1 hour ago
Concerns mount over retroactive taxation of banks’ profit in finance bill 1 hour ago, to curb market distortions, bridge exchange premium gap, cbn approves additional fx sale to bdcs at n1,450/$ 1 hour ago, breaking: fg, labour settled for n70,000 minimum wage, says minister 15 hours ago, latest headlines.

At Last, FG, Labour End Weeks of Feud, Settle for N70,000 National Minimum Wage

Concerns Mount over Retroactive Taxation of Banks’ Profit in Finance Bill

To Curb Market Distortions, Bridge Exchange Premium Gap, CBN Approves Additional FX Sale to BDCs at N1,450/$

Group to Tinubu: Review Tax, Pension Policies
Okonjo-iweala tells wto members to ‘walk the talk,’ engage in genuine negotiation.

The Director-General of the World Trade Organisation (WTO), Dr. Ngozi Okonjo-Iweala, has urged members of the global trade body to adopt a spirit of compromise and engage in genuine negotiation in order to overcome differences and reach agreements on several key issues at a General Council meeting scheduled for July 22nd and 23rd.
According a statement, reporting in her capacity as Chair of the Trade Negotiations Committee (TNC), Okonjo-Iweala, told members that while they were “a bit stuck” in many of the key issues, she was glad to hear an acknowledgment from a heads of delegation retreat held July 8, on the need to truly restart negotiating at the WTO.
“Now is the time to walk the talk and move from reflection and brainstorming to action,” she declared. “To not only take, but to be prepared to give. To compromise, be flexible and open-minded. To reach out to others, to understand their concerns and find mutually agreeable ways forward.
“There is engagement, but engagement is not necessarily negotiation,” she added.
“If we are to remain resilient at the WTO and responsive in a changing world, then we must remain faithful to the organisation’s negotiating DNA,” she said.
Prior to the Director-General’s report, members at the TNC meeting heard reports from the chairs of the negotiations on trade-related aspects of intellectual property rights (establishment of a multilateral register for geographical indications for wines and spirits), agriculture, development and fisheries.
The reports on the state of play in their negotiations were delivered by Ambassador Alfredo Suescum (Panama), Ambassador Alparslan Acarsoy (Türkiye), Ambassador Kadra Hassan (Djibouti) and Ambassador Einar Gunnarsson (Iceland), respectively.
On agriculture, Acarsoy noted efforts under way to define a roadmap for the continuation of the agriculture negotiations, referring to a number of member-led efforts.
“These include the Brazil-led process initiated a few months ago seeking a decision at the 22-23 July General Council on how to move the agriculture negotiations forward, the recent submission by the African Group introduced at the last meeting of the negotiating group on agriculture, and technical work being undertaken by the African Group, the Cairns Group of agricultural exporting countries and several other members, with a view to developing draft modalities in the different pillars of the agriculture negotiations,” he added.
Acarsoy said he would wait for the outcome of the Brazil-led process, taking also into account the recent African Group communication, before resuming the negotiating process after the summer break.
On development, Hassan said she had been working with a facilitator, Mr. Jia Jie Loh of Singapore, on how best to take work forward on the basis of the Ministerial Declaration adopted at MC13 on implementation of special and differential treatment provisions in the Agreement on Sanitary and Phytosanitary Measures and the Technical Barriers to Trade Agreement. The facilitator held an informal open-ended discussion with members on 10 July based on his bilateral consultations, the chair noted.
Hassan also noted that the facilitators dealing with the issues relating to the Agreement on Trade-Related Investment Measures and Article 66.2 of the Agreement on Trade-related Aspects of Intellectual Property Rights (TRIPS) relating to technology transfer have been reaching out to members on how to advance the work in their respective areas.

Related Articles

Agreement on Fisheries Subsidies: Okonjo-Iweala Receives Russia’s Instrument of Acceptance
Okonjo-iweala: people everywhere are feeling anxious about the future.

Okonjo-Iweala: I’m Too Busy Now to Think about Second Term
Climate crises: okonjo-iweala urges developing countries to prepare for ‘trips’ flexibilities.

Founded on January 22, 1995, THISDAY is published by THISDAY NEWSPAPERS LTD., 35 Creek Road Apapa, Lagos, Nigeria with offices in 36 states of Nigeria , the Federal Capital Territory and around the world. It is Nigeria’s most authoritative news media available on all platforms for the political, business, professional and diplomatic elite and broader middle classes while serving as the meeting point of new ideas, culture and technology for the aspirationals and millennials. The newspaper is a public trust dedicated to the pursuit of truth and reason covering a range of issues from breaking news to politics, business, the markets, the arts, sports and community to the crossroads of people and society.
Helpful Links
- Privacy Policy
- Terms & Conditions
You can email us at: [email protected] or visit our contact us page.
Denmark Joins Other EU States in Protest at Orban's Russia Trip
Denmark Joins Other EU States in Protest at Orban's Russia Trip

FILE PHOTO: Danish Foreign Minister Lars Lokke Rasmussen speaks during a press conference after a bilateral meeting at Eigtveds Pakhus in Copenhagen, Denmark, April 15, 2024. Ritzau Scanpix/Ida Marie Odgaard/via REUTERS/File Photo
COPENHAGEN (Reuters) - Denmark's foreign minister said on Thursday the country would not send ministers to informal government meetings linked to Hungary's EU presidency this month, in protest at Prime Minister Victor Orban's talks with the presidents of Russia and China.
Orban held talks on a potential Ukraine peace deal with Russian President Vladimir Putin and Chinese President Xi Jinping on separate occasions earlier this month, angering some EU leaders who warned against appeasing Moscow and said Orban did not speak for the 27-nation bloc.
The European Parliament on Wednesday adopted a resolution that condemned Orban's Russia visit, stating that the EU assembly "considers that this violation should be met with repercussions for Hungary".
Sweden Finland, Estonia, Latvia, Lithuania and Poland also last week said they would only be represented by civil servants.
"The government wants to clearly distance itself from the Hungarian presidency's handling of Ukraine in the first weeks of the Presidency," Foreign Minister Lars Lokke Rasmussen said in a written response to the Danish parliament's European Affairs Committee.
Denmark will, for now, only send civil servants to cover informal ministerial meetings in Hungary, he added.
(Reporting by Louise Breusch Rasmussen, editing by Sharon Singleton)
Copyright 2024 Thomson Reuters .
Photos You Should See - July 2024

Join the Conversation
Tags: Ukraine , Russia , Hungary , European Union , Denmark , Europe
America 2024

U.S. News Decision Points
Your trusted source for the latest news delivered weekdays from the team at U.S. News and World Report.
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

The Best Cartoons on Donald Trump
July 18, 2024, at 3:05 p.m.

Joe Biden Behind The Scenes
July 16, 2024

A Kinder, Gentler Trump?
Lauren Camera July 19, 2024

The End of Biden’s Reelection Bid?
Aneeta Mathur-Ashton July 18, 2024

20 Years of Secret Service Scandals
Aidan Pittman July 18, 2024

What We Know About Trump’s Shooter
Aneeta Mathur-Ashton and Alan Kronenberg July 18, 2024

The Biggest Moments of the RNC So Far
Laura Mannweiler July 18, 2024

Economy Slowing but Not in Recession
Tim Smart July 18, 2024

3 Takeaways From J.D. Vance's RNC Speech

Politics latest: Starmer announces £84m to 'grip' migration 'crisis' after summit with European leaders
Sir Keir Starmer pledges to "deepen" UK connection with the EU following European Political Community summit at Blenheim Palace. The prime minister announced new funding for humanitarian aid. Meanwhile, the Covid Inquiry concluded government 'failed' to prepare for the pandemic.
Thursday 18 July 2024 20:26, UK
- General Election 2024
Please use Chrome browser for a more accessible video player
- Sir Keir Starmer announces extra money for humanitarian support in order to 'grip' migration crisis, following summit with European leaders
- The COVID Inquiry released a report on UK preparedness for the pandemic
- Ed Davey and Pat McFadden gave evidence at the Post Office inquiry
- Beth Rigby: How to survive a general election
- Listen: What are Labour's key plans?
- Jon Craig : Sunak apologises to Tory MPs eight times at party 'wake'
- Live reporting by Faith Ridler and (earlier) Tim Baker
That's it from the Politics Hub today so thanks for joining us.
Don't forget to tune in to our special documentary tonight, Election: Behind The Scenes At Sky News, airing at 9pm on Sky Documentaries and 10.30pm on Sky Showcase.
You can catch it at 9pm tomorrow night on Sky News too.
Have a good evening!
Finally, we talk about MPs being told to smarten up in the Commons.
Earlier, Speaker Sir Lindsay Hoyle said male members needed to "wear a tie or you just might not catch my eye" if they want to speak in a debate.
So how important is it?
Labour's John McDonnell quotes a party colleague, saying: "It's not the smartness of the clothing but the smartness of the decisions you make."
But Conservative Alicia Kearns says they discuss "the most solemn of issues" in the chamber, so there is "something about the respect to the mother of all parliaments" in dressing smartly.
Now we turn to the director of the thinktank Civil Exchange Caroline Slocock to discuss the first report from the official COVID inquiry.
It was published today , and said UK citizens were "failed" by their governments' processes, planning and policy leading up to the pandemic.
Ms Slocock, who was a senior civil servant who worked under both Margaret Thatcher and John Major, says the report is "humbling" for government and the civil service, and "speaks to a failure of government".
She adds: "It's time for governments really to think differently about how they plan. I know from, not just from working with those two prime ministers, but also my wider experience of government that it's not very good at thinking ahead or thinking preventatively.
"[Government] tends to be better at crisis management and new initiatives than it is really managing the sorts of underbelly of issues which if you don't get that right, things come back to bite you."
Ms Slocock also echoed the report in her condemnation of so-called "group think" in Whitehall.
She said ministers do not "listen enough to people outside of central government", and that is "vital" in a situation like a pandemic.
Now we move over to our panel, Labour's John McDonnell and Conservative Alicia Kearns.
Ali Fortescue asks Mr McDonnell whether he ever imagined Donald Trump would get back into the White House.
He says after 6 January insurrection, he thought "America had learned his lesson with regard to Trump and his attitude towards the law, the constitution, democracy."
And he says it's "the worst nightmare they could possibly have" in America for its politics.
Ms Kearns agrees with her Labour counterpart over his concerns about what a Trump presidency means for Ukraine too.
Calling it a "grave concern", she added: "It is important we have a partner we can rely on for going forward to Ukraine."
Ali Fortescue then asks Labour's Nick Thomas-Symonds about the looming presence of the US election and the impact a Donald Trump victory could have on support for Ukraine.
Was it talked about at the EPC summit?
"Well we've been talking here about Europe," he tells Ali. "We've been talking about European solidarity backing President Zelenskyy and Ukraine.
"In respect of the American presidential election, that is a matter for the American people to decide, whoever they elect as their president.
"We as the UK government will work with them. We have a special relationship, a deep special relationship forged in the most difficult of historic circumstances, and I've no doubt that will endure into the future."
But surely European politicians have concerns, especially after the announcement of JD Vance as his running mate - who has been very critical of spending American money on a war in Europe.
"Well, let's let's just see what happens," says Mr Thomas-Symonds. "I'm not going to start going into hypotheticals, starting to anticipate what may or may not happen.
"What happens here is that the American people have a decision to make. They make that decision. We will respect that decision.
"We will always act in the UK's national interest and work with whoever is the president of the United States, because that is what is in Britain's national interest. And that's precisely what this government will do."
The government's minister for European relations Nick Thomas-Symonds is now talking to Ali Fortescue after what he deemed a successful day at the European Political Community summit at Blenheim Palace.
He points to the announcement made by Sir Keir Starmer to fund £84m of projects in Africa and the Middle East to tackle illegal migration at "source" and stopping people feeling the need to flee from their homes in the first place.
But Ali asks him when the public will see if the plan is working.
"Well, one person who is in those boats putting their lives at risk is one too many," he says. "So we are starting that work now.
"Of course you will judge, as will the British public judge, its success or failure on whether it is effective or not, whether it does actually crack down on this vile trade in people smuggling.
"And of course, you'll be able to see evidence of that as the government starts to implement its policy."
But he warns it could be a long wait, adding: "We have to bear in mind as well the really dire nature of our inheritance and the scale of this challenge.
"We know the scale of this challenge. We know how difficult it is, but we are determined to do the work to tackle it."
We start with our political editor Beth Rigby talking to us from outside Blenheim Palace, where the European Political Community (EPC) summit has been taking place.
She says the tone has been "very positive", with lots of diplomats and officials on the sidelines telling her "the reset is real and that the Labour government is really making progress".
The prime minister kicked off the conference by reassuring the European leaders that he would not leave the European Convention on Human Rights, says Beth - something often threatened by the former Conservative government.
"I'm told by one EU diplomat that this went down extremely well, that European leaders were not expecting it, but they really welcomed it and they saw it as proof, if you like, that Keir Starmer really does want to reset relations."
But while our political editor says "the reset is on", changing the relationship is "going to take time".
"Sir Keir said himself that it will be a combination of bilateral talks and then dealing with the European Union as an institution," she adds.
"But the mood music at Blenheim Palace was extremely positive. And Sir Keir and his team are very pleased with how things have gone down."
Now it's time for the Politics Hub with Ali Fortescue in the chair tonight.
Ali will be talking to the director of the Civil Exchange thinktank Caroline Slocock about the first report from the official COVID inquiry.
From the government, she will also speak to the minister for European relations Nick Thomas-Symonds .
And on the panel is Labour's former shadow chancellor John McDonnell and the Tory chair of the foreign affairs committee Alicia Kearns .
You can watch the show in the stream above, or follow our updates for all the highlights.
It may have been two weeks since Labour was voted into power, but they are still filling roles on their frontbench.
We have just had a list through of some new appointments, so take a look below...
- Hamish Falconer - parliamentary under secretary of state in the Foreign, Commonwealth and Development Office;
- Baroness Chapman - parliamentary under secretary of state in the Foreign, Commonwealth and Development Office;
- Mary Creagh - parliamentary under secretary of state in the Department for Environment, Food and Rural Affairs;
- Martin McCluskey - assistant government whip;
- Kate Dearden - assistant government whip.
By Jennifer Scott, political reporter
More than 192,000 children in England and Wales have parents who are currently in jail, new figures have revealed.
It is the first time the government has shown the scale of young people impacted - an estimated 192,912 - who, without support, often follow their parents into crime, studies show.
But charities say releasing the statistics is only "the first step", and are now calling for the new government to do more to support the "invisible children" who are "falling through the cracks".
Labour has a history of campaigning on the issue in opposition, with the now energy minister Kerry McCarthy bringing forward a private members' bill, calling for national guidelines, ensuring the state identifies any children at the point of sentencing, and accountability for giving them support.
The thrust of her bill made it into Labour's general election manifesto, which said: "The children of those who are imprisoned are at far greater risk of being drawn into crime than their peers. We will ensure that those young people are identified and offered support to break the cycle."
However, the commitment failed to appear in the King's Speech on Wednesday, which outlined the government's policy agenda for the next 12 to 18 months.
Read more below:
Be the first to get Breaking News
Install the Sky News app for free


IMAGES
VIDEO
COMMENTS
At MC12, trade ministers adopted the Ministerial Decisionon the TRIPS Agreement, which gives members greater scope to take direct action to diversify production of COVID-19 vaccines and to override the exclusive effect of patents through a targeted waiver over the next five years. It addresses specific …
We would like to show you a description here but the site won't allow us.
On June 17, 2022, the World Trade Organization (WTO) Ministerial Conference issued a "Draft Ministerial Decision on the TRIPS Agreement," addressing the requirements established in Articles 31 and 39.3 of the TRIPS Agreement in the context of the COVID-19 pandemic. 1 The Decision provides several clarifications regarding the process by ...
The WTO's Ministerial Decision on the TRIPS Agreement, adopted on June 17, 2022, provides a five-year waiver of certain requirements concerning CLs for COVID-19 vaccines.35 The decision permits an eligible member to use the subject matter of a patent, including ingredients and.
Early Friday morning, 17 June 2022, the WTO's 12th Ministerial Conference (chaired by Timur Suleymenov, Kazakshstan), adopted a Ministerial Decision on the TRIPS Agreement (WT/MIN (22)/W/15/Rev.2). The text can be found here. There were several changes since the June 10 version. The agreement is a limited and disappointing outcome overall ...
As part of the Administration's comprehensive effort to combat the pandemic, the United States supported negotiations that resulted in the WTO issuing the Ministerial Decision on the TRIPS Agreement on June 17, 2022. Since then, USTR officials consulted with Members of Congress and more than two dozen stakeholders, including public health ...
MINISTERIAL DECISION ON THE TRIPS AGREEMENT (WTO)* [June 17, 2022] WT/MIN(22)/W/15/Rev.2 17 June 2022 (22-4709) Ministerial Conference Original: English Twelfth Session Geneva, 12-15 June 2022 DRAFT MINISTERIAL DECISION ON THE TRIPS AGREEMENT Revision The Ministerial Conference,
Features of the MC12 TRIPS Decision that are less favourable vis-à-vis existing options to export under a compulsory licence under TRIPS. Use limited to developing country Members: Unlike in Article 31 and Article 31bis, only developing country Members of the WTO are eligible to use the Decision either as an importing or as an exporting Member ...
In February 2024, the 13th World Trade Organization (WTO) Ministerial Conference will take place. WTO Members are expected to decide whether to extend the June 17 2022 Ministerial Decision on the TRIPS Agreement, which is currently aimed at increasing access to Covid-19 vaccines, to therapeutics and diagnostics. The original deadline for this decision was December of 2022.
Ministerial Decision on the TRIPS Agreement, 17 June 2022 . Paragraph 8 of the Ministerial Decision on the TRIPS Agreement, 17 June 2022 . Agenda item 3 and 11 Decision WT/L/1141 . Following the negotiations on the proposal by developing country members for a TRIPS waiver, the 12th WTO Ministerial Conference on 17 June 2022 adopted a ...
On 17 June 2022, the WTO Ministerial Conference adopted a long-awaited decision on the TRIPS Agreement. The Decision has not waived any intellectual property rights as such, as proposed by India and South Africa in October 2020. Instead, it mainly clarifies the application of the existing TRIPS flexibilities. As a follow-up to its earlier Position Statement, the Institute issued a paper that ...
ABU DHABI, 1st March, 2024 (WAM) -- The WTO 13th Ministerial Conference today approved an extension to the TRIPS Waiver, enabling developing countries to increase vaccine production capacity for COVID-19 and future pandemics. The decision was one of the key objectives of the 13th WTO Ministerial Conference.
Finally, on 17 June 2022, during the 12th WTO Ministerial Conference (MC12), the Ministerial decision on the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement, aka the TRIPS Waiver, was a fact. The decision entails a strongly watered-down version of the original TRIPS Waiver drafted by India and South Africa.
The resulting "Quad proposal" eventually provided the basis for negotiating the Ministerial Decision on the TRIPS Agreement at the Twelfth WTO Ministerial Conference in Geneva in June 2022. This decision allowed WTO members to manufacture without the authorization of patent holders COVID-19 vaccines—and, if subsequently approved, also other ...
The 12th WTO Ministerial Conference adopted a Ministerial Decision on the TRIPS Agreement on 17 June 2022. This partially concluded almost two years of protracted discussions in response to a proposal by India and South Africa for a waiver from certain obligations under the TRIPS Agreement for health products and technologies for the prevention ...
Closing session of the 12th WTO ministerial conference in Geneva, Switzerland. Photo by: Fabrice Coffrini / Pool via Reuters. After its four-day ministerial conference spilled over into a sixth ...
A new draft ministerial decision spearheaded by the WTO Director General (WT/MIN(22)/W/15) but largely based on proposals from the European Union, was discussed and eventually adopted at the WTO ...
On June 17, 2022, the World Trade Organization (WTO) Ministerial Conference issued a "Draft Ministerial Decision on the TRIPS Agreement," addressing the requirements established in Articles 31 ...
This decision allowed WTO members to manufacture COVID-19 vaccines—and, if subsequently approved, also other COVID-19 health products—without the authorization of the relevant patent holders. This chapter traces the TRIPS waiver debate from the submission of the original proposal by India and South Africa in October 2020 to the final ...
Responding to today's ministerial decision by the World Trade Organization (WTO) on the TRIPs Agreement, Tamaryn Nelson, Amnesty International's Researcher on Economic, Social and Cultural Rights, said: "More than two years into the Covid-19 pandemic and the WTO still hasn't made the changes needed to ensure everyone has access to life ...
The Model WTO is the biggest and most complete simulation of WTO's Ministerial negotiations in the world. The Model WTO provides a unique opportunity for future decision makers to experience the debates of the World Trade Organisation. Every year since 1997, students from around the world have been invited to Switzerland to debate and ...
The Model WTO is the biggest simulation of the World Trade Organization negotiations in the World. Our international conference provides a unique opportunity for future decision makers to experience first hand the technicalities of the multilateral trading system. Every year since 1997, students from around the world and different educational ...
The Director-General of the World Trade Organisation (WTO), Dr. Ngozi Okonjo-Iweala, has urged members of the global trade body to adopt a spirit of compromise and engage in genuine negotiation in ...
The European Commission has decided to boycott Hungary's six-month presidency of the EU Council in response to Viktor Orbán's controversial trips to Moscow and Beijing, widely seen as an affront ...
President Joe Biden addressed his 'bull's-eye' comment, took swings at the media and defended his decision to stay in the race. Laura Mannweiler July 15, 2024 Trump's New Rallying Cry: Unity
The General Council is the second highest decision-making body of the WTO after the ministerial conference (MC), which meets after every two years. The World Forum of Fisher Peoples and the World Forum of Fish Harvesters and Fish Workers have raised concerns over the current text and said that exemptions for small-scale fishers across ...
Sir Keir Starmer is hosting the European Political Community summit at Blenheim Palace in Oxfordshire. Small boats, immigration and the war in Ukraine are all expected to be discussed.