

The Prospects for an IP Waiver Under the TRIPS Agreement
By Duncan Matthews and Timo Minssen
The informal meeting of the World Trade Organization (WTO) Trade-Related Aspects of Intellectual Property Rights (TRIPS) Council today, July 6, 2021, focuses international attention once more on prospects for a waiver of the TRIPS Agreement in response to the COVID-19 pandemic.
Regardless of whether an actual TRIPS waiver ultimately comes to pass, the real significance of these efforts lies in the increased focus they have placed on the role of IP and trade secrets in improving access and affordability, and scaling-up of manufacturing and supply of vaccines and other health-related technologies. These conversations have introduced the possibility of a rethinking of the relationship between IP, innovation, conservation, and access.
The proposal for a TRIPS waiver, made initially by India and South Africa last October, was given additional impetus on May 5, 2021 when United States Trade Representative Katherine Tai announced that the U.S. supports a waiver for COVID-19 vaccines and will actively participate in text-based negotiations at the WTO to make that happen.
Since then India, South Africa, and their supporters issued revised draft text on May 25, 2021, which would waive (for an initial period of three years) IP on all relevant health products and technologies, including diagnostics, therapeutics, vaccines, medical devices, personal protective equipment, their materials or components, and their methods and means of manufacture for the prevention, treatment or containment of COVID-19.
On June 4, 2021, the EU issued its own proposal , which focuses not on an IP waiver, but instead calls for limits on export restrictions, voluntary measures to encourage and support vaccine production and affordability, and the use of voluntary licenses as the most effective instrument to facilitate the expansion of production and sharing of expertise.
These proposals differ in the details, but share a common goal. They are both broad-based calls to find solutions to the complex question of how best to scale up manufacturing and supply, while improving access and affordability, in response to the COVID-19 pandemic.
Ultimately, however, the WTO is a member-driven institution, and agreement on a TRIPS waiver will require either consensus, or, if it were to go to a vote, a three-fourths majority in accordance with Article IX of the WTO Agreement . Currently, WTO members supporting the waiver simply don’t have the numbers to achieve this. About 123 WTO members would be needed if this went to a vote under Article IX of the WTO Agreement. Even optimistically, the current number of WTO members supporting the waiver is only half that total. In reality, when deciding on whether a consensus or majority approach will be sought, the Chair of the WTO General Council will have a great deal of discretion as to what will happen next. He will make that decision based on the information he receives from the Chair of the TRIPS Council, but there will be no vote taken at the TRIPS Council itself. Only the WTO Ministerial Conference (slated for November 30 – December 3 2021) can decide this. We do expect some sort of WTO Declaration on IP and COVID-19 to emerge by December 3, but whether this is anywhere close to current TRIPS waiver proposals remains to be seen. Watering down the current TRIPS waiver proposals to achieve a consensus or majority vote remains a very real possibility.
Meanwhile, the academic debate has crystallized around almost diametrically opposed pro-waiver and anti-waiver positions. Our view is that both positions have their merits and their drawbacks, but that, in reality, pro- and anti-waiver arguments are not binary.
Already, we have stressed this in our letter to the Financial Times , published on May 12, 2021, just days after the Biden Administration’s announcement of support for a waiver. We argue that debating IP waivers will fuel pandemic innovation (the original, unedited version of our letter is available here ).
In our longer Financial Times opinion of June 17, 2021, we then called for greater transparency of ownership, licensing, and advance purchase agreements to inform a better understanding of the effects of IP during the pandemic response (the original, unedited version of our opinion is available here ). As we state in the Financial Times , the COVID-19 response requires a toolkit of policies to ensure adequate vaccine manufacturing and delivery. The current focus on the role of IP can be part of that toolkit, and its interface with collaboration and knowledge transfer in responding to these challenges must be key components of that response.
Opinions differ on what long-term impact a potential IP waiver would have on pandemic responses and future innovation. There are also debates about whether it is the best tool to reach the necessary goals. Yet, it is understandable that the waiver is being considered as a possible approach for countries seeking to scale up manufacturing and supply and improve affordability.
However, regardless of whether a WTO waiver is ever achieved, it will not alone solve the access problem. More broadly, the debate about an IP waiver will focus attention on the ability for WTO members to apply pressure on the manufacturers of COVID-19 vaccines and related health care technologies. It will make clear that, while voluntary approaches are preferred, compulsory licenses, or even waiving IP remains the ultimate sanction if IP owners are not willing to transfer technology in order to scale up production, lower prices, and achieve more equitable distribution globally.
In many ways, the current focus on a TRIPS waiver is likely to create these pressure points, regardless of whether it realizes final agreement at the WTO Ministerial Conference. Attention is already focusing on rethinking manufacturing and improving supply chains, and this is likely to include: a greater willingness to engage in voluntarily licensing agreements, participation in technology access pools (C-TAP), greater transparency of Advance Purchase Agreements, greater transparency and sharing of regulatory data, mechanisms (including regulatory obligations and incentives) to increase transfer of tacit know-how, compulsory licensing, more inclusive and more comparable clinical trial designs , and greater recourse to competition law.
Ultimately, the world needs to find sustainable solutions to address not only the current crisis, but also to assist with future pandemic preparedness. These solutions may include, inter alia : (1) greater consideration of the role of regional manufacturing hubs, (e.g., the recent WHO announcement of support for the South African consortium to establish the first COVID mRNA vaccine technology transfer hub , which can assist with knowledge transfer to speed up manufacturing and supply), (2) collaboration between global organizations, such as the recent collaboration between the World Health Organization (WHO), World Intellectual Property Organization (WIPO), and WTO, (3) a WHO Pandemic Preparedness Treaty , and (4) a joint EU-U.S. COVID Manufacturing and Supply Chain Taskforce .
We recognize that some of these initiatives may not come to fruition, or may not contribute substantively to improved manufacturing, supply, and affordability, but they are steps in the right direction.
These steps are being taken not least because the TRIPS waiver proposal has focused attention on the role of IP during the pandemic. For this, the TRIPS waiver debate must be commended. Without it, the current debate would lack substantive focus. Going forward, practical solutions should focus on scaling up manufacturing and supply, and improving access and affordability, while maintaining the long-term objectives of the innovation system and contributing to future pandemic preparedness.
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to print (Opens in new window)

Timo Minssen
Timo Minssen is Professor of Law at the University of Copenhagen (UCPH) and the Founder and Managing Director of UCPH's Center for Advanced Studies in Biomedical Innovation Law (CeBIL). He is also affiliated with Lund University as a researcher in Quantum Law. His research concentrates on Intellectual Property, Competition & Regulatory Law with a special focus on new technologies in the pharma, life science & biotech sectors including biologics and biosimilars. His studies comprise a plethora of legal issues emerging in the lifecycle of biotechnological and medical products and processes - from the regulation of research and incentives for innovation to technology transfer and commercialization.
Leave a Reply Cancel reply
You must be logged in to post a comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Sign up for our newsletter
News & Insights
- North America
- Los Angeles
- Northern Virginia
- San Francisco
- Silicon Valley
- Washington, D.C.
- Europe & Middle East
- Capabilities
- Corporate, Finance and Investments
- Activist Defense
- Banking and Institutional Finance
- Capital Markets
- Construction and Procurement
- Corporate Governance
- Emerging Companies and Venture Capital
- Employee Benefits and Executive Compensation
- Energy and Infrastructure Projects
- Financial Restructuring
- Global Human Capital and Compliance
- Investment Funds and Asset Management
- Mergers and Acquisitions (M&A)
- Middle East and Islamic Finance and Investment
- Private Credit & Special Situations
- Private Equity
- Public Companies
- Real Estate
- Structured Finance and Securitization
- Technology Transactions
- Government Matters
- Data, Privacy and Security
- Environmental, Health and Safety
- FDA and Life Sciences
- Government Advocacy and Public Policy
- Government Contracts
- International Trade
- National Security and Corporate Espionage
- Securities Enforcement and Regulation
- Special Matters and Government Investigations
- Trial and Global Disputes
- Appellate, Constitutional and Administrative Law
- Bankruptcy and Insolvency Litigation
- Class Action Defense
- Commercial Litigation
- Construction and Engineering Disputes
- Corporate and Securities Litigation
- E-Discovery
- Intellectual Property
- International Arbitration and Litigation
- Labor and Employment
- Product Liability
- Professional Liability
- Toxic & Environmental Torts
- Industries / Issues
- Automotive, Transportation and Mobility
- Energy Transition
- Financial Services
- Food and Beverage
- Higher Education
- Life Sciences and Healthcare
- Artificial Intelligence and Machine Learning
- Buy American
- Crisis Management
- Doing Business in Latin America
- Environmental Agenda
- Environmental, Social and Governance (ESG)
- Focus on Women's Health
- Russia/Ukraine
- Special Purpose Acquisition Companies (SPACs)
- Experienced Lawyers
- Law Students
- UK Graduate Recruitment
- Judicial Clerks
- Other Professionals
- News & Insights
- Cases & Deals
- In the News
- Press Releases
- Recognitions
- Conferences
- Speaking Engagements
- All Insights
- Newsletters
- Client Alerts
- Thought Leadership
- Auditor Liability Bulletin
- ESG Excellence
- Diversity & Inclusion
- Women’s Initiatives
- Racial Diversity
- LBGTQ+ Affinity
- Citizenship
- Community Focus
Client Alert
June 7, 2021
Update on the Proposed TRIPS Waiver at the WTO: Where is it Headed, and What to Expect?
On June 8-9, 2021, the World Trade Organization’s (WTO) TRIPS Council will hold their first meeting in the wake of the U.S. Trade Representative (USTR) announcing “the Biden-Harris Administration’s support for waiving intellectual property protections for COVID-19 vaccines” under the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement). A TRIPS waiver, if issued, could potentially permit the unimpeded manufacture and distribution of COVID-19 vaccines, diagnostic kits, and therapeutics without regard for the intellectual property rights of the inventors and developers of the technologies that enabled their development, and without following existing effective processes for ensuring widespread access to life-saving immunizations, testing, and treatment.
Since the USTR’s announcement, 62 countries sponsoring a TRIPS waiver issued a revised proposal 1 Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19, TRIPS Communication IP/C/W/669/Rev.1 (May 21, 2021) to advance text-based negotiations; China expressed openness to discussing the waiver; Germany and others stated their continued opposition, and the EU has urged a “third way” proposal. The TRIPS waiver has also been disputed in U.S. Congress, with some opponents introducing legislation to limit USTR’s authority to agree to a waiver in the WTO. The public debate over the waiver has shifted as well – with an increasing focus on trade secrets, technology transfer, supply chain constraints, and other factors impacting global vaccine production aside from any effects of patent rights.
Continue reading for our update on where the TRIPS waiver proposal is headed; the practical concerns raised by the proposal; and potential legal limitations that may impact passage and implementation of a TRIPS waiver. And see our previous client alert on the TRIPS waiver proposal for additional background and insights.
Where does the TRIPS waiver stand, and where is it headed?
In their original October 2, 2020 submission to WTO, India and South Africa proposed waiving member countries’ TRIPS obligations to protect any patents, copyrights, industrial designs, or trade secrets (“undisclosed information”) “in relation to prevention, containment or treatment of COVID-19,” until “widespread vaccination is in place globally.” 2 Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19, TRIPS Communication IP/C/W/669 (Oct. 2, 2020)
For months thereafter, the waiver proposal remained deadlocked in the WTO, largely with developing nations signing on to sponsor or support the waiver, and multiple developed nations opposing the waiver in favor of alternative approaches to increasing global vaccine equity. But on May 5, 2021, USTR Katherine Tai made the unprecedented announcement that the Biden-Harris Administration “supports the waiver of [intellectual property] protections for COVID-19 vaccines,” and “will actively participate in text-based negotiations at the [WTO] needed to make that happen.” 3 See Statement from Ambassador Katherine Tai on the COVID-19 TRIPS Waiver (May 5, 2021), available at https://ustr.gov/about-us/policy-offices/press-office/press-releases/2021/may/statement-ambassador-katherine-tai-covid-19-trips-waiver
Since then, international positions surrounding the waiver proposal have been evolving at a rapid pace – with new developments reported almost daily. China announced on May 18 that it would support a TRIPS waiver “that is conducive to fair access to vaccines in developing countries” 4 See Inside Health Policy, Tai Talks TRIPS Waiver with Allies as China Gets Behind it, GOP Balks (May 20, 2021), available at https://insidehealthpolicy.com/daily-news/tai-talks-trips-waiver-allies-china-gets-behind-it-gop-balks ; Germany expressly stated opposition to a TRIPS waiver; and the EU more broadly announced support for a “third way” alternative that includes “trade facilitation and disciplines on export restrictions,” “support for the expansion of production” (including through voluntary licensing agreements), and “clarifying and simplifying the use of compulsory licenses [under TRIPS] during crisis times.” 5 See Health Policy Watch, G20 Leaders Promise to Share More Vaccines While EU Digs in Against TRIPS Waiver (May 21, 2021), available at https://healthpolicy-watch.news/g20-leaders-promise-to-share-more-vaccines-while-eu-digs-in-against-trips-waiver/; European Commission, Opening Statement by Executive Vice-President Valdis Dombrovskis at the European Parliament plenary debate on the Global COVID-19 challenge (May 19, 2021), available at https://ec.europa.eu/commission/commissioners/2019-2024/dombrovskis/announcements/opening-statement-executive-vice-president-valdis-dombrovskis-european-parliament-plenary-debate_en; Bloomberg, EU’s Trade Response to Pandemic Stops Short of Vaccine IP Waiver, available at https://www.bloomberg.com/news/articles/2021-06-03/eu-s-trade-response-to-pandemic-stops-short-of-vaccine-ip-waiver WTO Director-General Ngozi Okonjo-Iweala, who has supported a “third way” in recent months, 6 See Statement of Director-General Elect Dr. Ngozi Okonjo-Iweala to the Special Session of the WTO General Council (Feb. 13, 2021), available at https://www.wto.org/english/news_e/news21_e/dgno_15feb21_e.pdf now states that “we must act now to get all our ambassadors to the table to negotiate a [waiver] text.” 7 See supra note 5
Notably, the USTR’s support for a waiver targeting “COVID-19 vaccines ” differs substantially from the May 21, 2021 revised proposal by India, South Africa and their 60 co-sponsors – which relates broadly to “ health products and technologies … for the prevention, containment or treatment of COVID-19.” Under the sponsors’ revised proposal, WTO members would be relieved (for at least 3 years) of their TRIPS obligations to protect patents, copyrights, industrial designs and trade secrets for any “ diagnostics, therapeutics, vaccines, medical devices, personal protective equipment ” used in relation to preventing, containing or treating COVID-19 – as well as “ their materials or components, and their methods and means of manufacture .” Notably, the revised proposal is not substantially different from the original proposal, and suggests that WTO members may remain divided on this controversial issue.
The next meeting of the TRIPS Council to discuss the waiver proposal is taking place on June 8-9, 2021, and will be closely watched. Because any TRIPS waiver would require consensus of all 164 WTO member countries, any text-based negotiations that take place would likely proceed over the course of several months or more.
Proponents have advanced the proposed TRIPS waiver in the name of meeting global vaccine demand. But even in the absence of a waiver, pharmaceutical manufacturers have continued efforts to expand global production and distribution of COVID-19 vaccines and therapies, with a focus on expanding access to developing countries. For example, Pfizer announced its plan to deliver two billion doses to developing nations over the next 18 months, with one billion doses coming this year. 8 Wall Street Journal, Pfizer, BioNTech to Deliver 2 Billion Covid-19 Vaccine Doses to Developing Countries (May 21, 2021), available at https://www.wsj.com/livecoverage/covid-2021-05-21/card/GsPYoFscRppTzYYt0l4f One forecast estimates that, by the end of 2021, total global COVID-19 vaccine production may exceed 11 billion doses – an amount potentially sufficient to achieve global herd immunity. 9 Airfinity, How Much Vaccine Will be Produced This Year? (May 19, 2021), available at https://www.airfinity.com/insights/how-much-vaccine-will-be-produced-this-year
Several pharmaceutical industry groups have also proposed a five-step plan to “urgently advance COVID-19 equity,” including: (1) increasing dose sharing among countries through COVAX and other mechanisms; (2) optimizing production of vaccines and raw materials; (3) eliminating trade barriers for critical raw materials; (4) supporting country readiness to deploy vaccination programs; and (5) driving further innovation. 10 IFPMA, Five steps to urgently advance COVID-19 vaccine equity (May 19, 2021), available at https://www.ifpma.org/resource-centre/five-steps-to-urgently-advance-covid-19-vaccine-equity/
Manufacturers have also continued to partner with other companies in efforts to scale up global production. For example, Moderna recently engaged Samsung Biologics to provide fill-and-finish manufacturing for Moderna’s vaccine. 11 Samsung Biologics, Moderna and Samsung Biologics Announce Agreement for Fill-Finish Manufacturing of Moderna's COVID-19 Vaccine (May 22, 2021), available at https://www.prnewswire.com/news-releases/moderna-and-samsung-biologics-announce-agreement-for-fill-finish-manufacturing-of-modernas-covid-19-vaccine-301297280.html Merck and Gilead also each entered into or expanded voluntarily licensing programs with manufacturers in India to produce the companies’ respective COVID-19 antiviral agents molnupiravir and remdesivir. 12 Fierce Pharma, Gilead, Merck step in to help India's drug manufacturers fight surging COVID-19 outbreak (Apr. 27, 2021), available at https://www.fiercepharma.com/pharma/gilead-merck-plan-production-boosts-for-covid-19-drugs-india-amid-surging-outbreak
Some WTO members have also considered using the existing TRIPS flexibilities to expand their vaccine access. For example, Bolivia has continued to pursue its effort to import the Johnson & Johnson COVID-19 vaccine from Canadian company Biolyse Pharma, under a compulsory license pursuant to TRIPS Article 31 bis (if one could be obtained). 13 WTO News, Bolivia outlines vaccine import needs in use of WTO flexibilities to tackle pandemic (May 12, 2021), available at https://www.wto.org/english/news_e/news21_e/dgno_10may21_e.htm
How might a TRIPS waiver impact ongoing efforts to scale COVID-19 vaccine production?
A TRIPS waiver would potentially hurt, not help, the current efforts to expand global production of COVID-19 vaccines and therapies. For example, expanding vaccine production to unlicensed manufacturers could further exacerbate the challenging supply chain issues and high demand for limited raw materials that have hindered even the authorized vaccine manufacturers from scaling up production in recent months. 14 See, e.g. , EndPoints News, As fears mount over J&J and AstraZeneca, Novavax enters a shaky spotlight (Apr. 21, 2021), available at https://endpts.com/as-fears-mount-over-jj-and-astrazeneca-novavax-enters-a-shaky-spotlight/; New York Times, U.S. to Send Virus-Ravaged India Materials for Vaccines (Apr. 25, 2021), available at https://www.nytimes.com/2021/04/25/us/politics/india-us-coronavirus.html
A TRIPS waiver could also disincentivize the current vaccine manufacturers from entering into further voluntary licensing agreements with manufacturing partners in other countries. In the wake of the USTR’s announcement, supporters of a TRIPS waiver have shifted the public discussion from patents to confidential trade secrets and technology transfer – acknowledging that waiving patent rights would not be sufficient to expand global production of safe and effective COVID-19 vaccines. 15 Financial Times, Biden Urged to Oblige US Vaccine Makers to Share Technology (May 15, 2021), available at https://www.ft.com/content/9408223f-0a6c-43b7-9f67-c7e4697005c2 But, in the absence of voluntary licensing agreements, there is no clear mechanism for countries to compel the original vaccine manufacturers to divulge trade secrets or provide technology transfer support to unlicensed parties. And if pharmaceutical companies perceive that their technology transfer to a voluntary licensee could lead to the licensee’s country disseminating their competitively sensitive know-how, they might forgo such voluntary licensing entirely.
Likewise, global pharmaceutical development could be seriously harmed if WTO members use a TRIPS waiver to divulge the vaccine manufacturers’ confidential trade secrets submitted during regulatory review – information that TRIPS generally protects from disclosure and unfair commercial use by unauthorized parties. 16 See TRIPS Agreement, Article 39.3 If pharmaceutical companies perceive that submitting to regulatory review in certain countries means losing protections for proprietary manufacturing processes and other competitively sensitive trade secret information, those companies might consider avoiding such countries altogether when considering where to develop, make, sell, or license their products.
Finally, the TRIPS waiver could negatively impact the continued development of COVID-19 vaccine. There are more than 60 additional vaccines under development worldwide; and for many of these efforts, the investment of time and money is premised on the potential for licensing the vaccine or partnering with a larger manufacturer to produce it. Prospectively waiving intellectual property protections for these vaccines in development will likely undermine continued investment and research – a proposition made all the more alarming due to the possibility of new and different strains of COVID-19.
What legal factors might prevent passage or limit implementation of a TRIPS waiver?
The most immediate hurdle to passage of a TRIPS waiver lies in the requirement that all 164 WTO member countries agree to a specific waiver text. As noted above, Germany (among others) presently objects to any waiver proposal; the U.S. has only stated support for a waiver of significantly narrower scope than the sponsors’ current proposal; and a “third way” proposal that sidesteps a TRIPS waiver remains on the table. And in the U.S., legislation has been proposed in Congress to limit the USTR’s authority to agree to a TRIPS waiver, e.g. , by requiring Congressional approval of any waiver, 17 See, e.g. , H.R. 3236, 117th Cong. or prohibiting the use of federal funds to support a waiver. 18 See, e.g. , H.R. 3035, 117th Cong.; S. 1683, 117th Cong. One of the proposals in the Senate was narrowly voted down, but garnered some bipartisan support. We expect that Congressional interest will remain high on this topic, and there may be a pathway to bipartisan action that would constrain the Administration’s waiver of IP protections under TRIPS.
Even if a TRIPS waiver of some scope were passed in the WTO after text-based negotiations, hurdles would remain to its effective implementation. A TRIPS waiver would not change the applicable IP protections in the WTO member states; and each member state would need to decide on their own (through their individual lawmaking procedures) whether and how to change their domestic laws within the scope permitted by the TRIPS waiver. It is unlikely that the resulting global patchwork of inconsistent IP protections would facilitate further expansion of vaccine production – particularly if voluntary technology transfer from existing manufacturers remains critical to making safe and effective vaccines at scale.
To that end, some waiver proponents have called on the U.S. to compel technology transfer from the U.S.-based vaccine manufacturers. 19 See supra note 15 But current U.S. statutes largely prohibit the FDA and other regulatory agencies from publicly divulging trade secret information submitted for purposes of regulatory approval. 20 See, e.g. , 21 U.S.C. § 331(j); 18 U.S.C. § 1905; 5 U.S.C. § 552(b)(4); Chrysler Corp. v. Brown , 441 U.S. 281 (1979) And even if authorized by future legislation, the Taking Clause of the Fifth Amendment would likely prohibit the U.S. government from disclosing trade secret information submitted under the current statutory and regulatory protections, without just compensation ( e.g. , damages awarded against the U.S. under the Tucker Act by the Court of Federal Claims). 21 See, e.g. , Ruckelhaus v. Monsanto Co. , 467 U.S. 986 (1984); 28 U.S.C. § 1491
Additionally, even in the wake of a TRIPS waiver, companies subject to unauthorized use of their COVID-19 vaccine or therapy IP outside the U.S. may have remedies available under international law. Even though the type of obligations included in TRIPS are also included in certain U.S. trade agreements, such as the United States-Canada-Mexico Agreement (the “USMCA”), it is highly unlikely that the current U.S. administration will seek to invoke state-to-state dispute settlement on this matter given their support of the waiver proposal. 22 See USMCA Ch. 20. Morever, in the event of a TRIPS waiver, the USMCA provides that “the Parties shall immediately consult in order to adapt this [agreement] as appropriate.” USMCA Art. 20.6(c)
IP rights holders can also consider the availability of investor-state dispute settlement or other arbitration procedures. For example, affected holders of IP rights might be able to bring claims for compensation against WTO member states under any applicable bilateral investment treaties (BITs) – to the extent that such treaties do not contain express exceptions to IP‑related rights under the TRIPS Agreement. Foreign investors may be able to claim that such states had committed to providing greater protections under any applicable BIT, such that these protections cannot be displaced by any waiver under the TRIPS Agreement. That said, such states – particularly those with limited resources – may try to rebut any claims of wrongfulness by asserting an ongoing state of necessity for public health emergencies during the COVID‑19 global pandemic. The strength of a potential claim under a BIT would be fact-specific for each IP rights holder, and should be assessed individually.
* * *
In light of continuing developments at the WTO, companies that hold intellectual property in medical products related to COVID-19 should seek the advice of counsel to develop legal and policy strategies regarding the TRIPS waiver request, including (1) if they receive requests for licensing authorization, (2) if they learn of unauthorized use of their intellectual property, or (3) if they engage with national governments or international organizations to advocate for desired policy outcomes.
Featured Lawyers
Jamieson L. Greer Washington, D.C.
Jeffrey M. Telep (Jeff) Washington, D.C.
Daniel Crosby Geneva
Brian A. White Atlanta
Related Documents
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
WTO TRIPS Waiver for COVID-19 Vaccines
Sharing the know-how behind making COVID-19 vaccines is key to scaling up production and addressing emerging variants.
A Q&A WITH ANTHONY D. SO, MD, MPA
The Biden administration announced it would seek a “TRIPS waiver” of intellectual property protections related to COVID vaccines.
In this Q&A, Anthony D. So, MD, MPA , a professor of the practice in International Health , explains the waiver and what it could mean for COVID-19 vaccines.
What’s the TRIPS waiver for COVID vaccines all about?
The TRIPS waiver refers to a proposal, advanced by the governments of South Africa and India, to the World Trade Organization to waive intellectual property rights protection for technologies needed to prevent, contain, or treat COVID-19 “until widespread vaccination is in place globally, and the majority of the world’s population has developed immunity.”
In 1995, when the World Trade Organization came into existence, those signing up as members agreed that in exchange for the lowering of barriers to trade, they would abide by the Agreement on Trade-Related Aspects of Intellectual Property Rights, or TRIPS.
This Agreement, pushed by knowledge-based economies like the United States and the multinational, research-intensive pharmaceutical industry, imposed a base of protections for intellectual property rights, from patents to copyrights. Before this was negotiated, more than 50 countries did not recognize patent protection on pharmaceutical products. The TRIPS Agreement changed that, and after a transition period of 10 years, ratcheted up these requirements on all but the least developed countries.
Middle-income countries like India came into compliance by 2005. The TRIPS waiver just seeks to temporarily suspend these protections until the pandemic has ended, so the world can better access the knowledge needed to combat the worst pandemic in a century.
What’s the rationale behind waiving intellectual property protections for COVID vaccines?
Sharing the know-how behind making COVID-19 vaccines is key to not only scaling up production, but also bringing forward the second generation of vaccines we will need to address emerging variants.
No single vaccine manufacturer can produce enough vaccines to cover the globe, and demand has far outstripped supply, with high-income countries taking the lion’s share of reserved doses. Proponents of a TRIPS waiver wonder how it can be right for a multinational vaccine manufacturer to hold exclusive rights that can stop other firms from stepping up to meet the need for vaccines, particularly in markets not being served by current vaccine producers. They argue that the public already has paid once or twice for such innovation, either upfront in research and development (R&D) costs or through purchase guarantees of these products, or both.
Moderna’s COVID-19 vaccine—one of two now in use based on an mRNA platform—was paid for largely by the U.S. government, and in fact, Moderna has pledged not to enforce its patents related to the COVID-19 vaccine during the pandemic. However, making the Moderna vaccine likely involves other companies’ patented equipment and processes as well, so waiving patent protections on one piece of the process may not help other companies make the entire “recipe.”
This is why a TRIPS waiver is considered important to ensure other vaccine manufacturers would have the freedom to operate. It should also be acknowledged that a TRIPS waiver may accelerate scaling up some COVID-19 vaccines where untapped capacity for vaccine production still exists, and it may also encourage existing vaccine producers to step up their technology transfer efforts.
By noting its willingness to move forward with text-based negotiations over a TRIPS waiver at the World Trade Organization, the United States signaled a seismic shift in policy. However, it is only the beginning of a process.
Who is opposing the TRIPS waiver and why?
Other high-income countries, from EU countries and the United Kingdom to Japan and Australia, have yet to join the United States in supporting negotiations for a TRIPS waiver. Multinational pharmaceutical companies have vocally opposed the waiver, and disclosure forms from the first quarter of 2021 reveal that over 100 lobbyists had been enlisted to oppose the TRIPS waiver.
The main arguments boil down to protecting the incentive for future pharmaceutical innovation. The idea is that companies will be reluctant to invest in new technology if they feel that they cannot reap full financial benefit from their successes.
Yet before COVID-19 hit, R&D for pandemic vaccines was largely neglected by the private sector, and public financing understandably had to support this work. Many of the first COVID-19 vaccines that came to market relied on tax dollars to enable their effective development.
One of the key aspects of producing successful COVID-19 vaccines has been the process of stabilizing the COVID-19 spike protein. This innovation relied largely on public funding, and all of the vaccines currently on the U.S. market rely on this technology. But although they all benefited from publicly funded intellectual property, no vaccine company has stepped forward to join a global effort to voluntarily share its know-how through the COVID-19 Technology Access Pool (C-TAP), launched by WHO with the support of Costa Rica and 40 member state cosponsors.
Another question raised by opponents of the waiver is: Have companies been able to make a strong return on their investment? The answer appears to be yes. In a market almost entirely created by public sector purchase of vaccines for a pandemic, Pfizer brought in $3.5 billion in COVID-19 vaccine revenues in the first quarter of this year, with estimated profit margins in the high 20% range, by far its greatest revenue generator. Pfizer’s partner, BioNTech, received upfront public financing, both from the German government and the European Investment Bank, while Pfizer itself has secured 6 billion dollars thus far from the U.S. government in guaranteed purchases of its COVID-19 vaccine. So even if the TRIPS waiver were to enable other vaccine producers to meet the huge unmet demand, it is hard to argue that the public sector has not already provided multibillion-dollar incentives to bring forward needed innovation.
The pharmaceutical industry has also argued that the TRIPS waiver will not speed the scale-up of COVID-19 vaccines. This argument reflects the fact that the production process is very complex and difficult to develop without extensive support from existing manufacturers.
This may be particularly true for mRNA technology. While mRNA vaccine candidates are emerging in India and China as well, the intellectual property landscape for mRNA vaccines is highly fragmented, with a handful of pharmaceutical companies holding half of these patent applications. The TRIPS waiver would clear the path for these firms to move forward, as they enter clinical testing, without concern over conflicting patent claims that may not be resolved at pandemic speed.
Unlike Pfizer and Moderna, which make mRNA vaccines, AstraZeneca/Oxford has struck many more technology transfer agreements with vaccine producers in low- and middle-income countries for its vaccine based on adenovirus vector technology, which is easier to produce and distribute. Its vaccine price of $3 per dose is also less than half that of Pfizer/BioNTech’s vaccine in the African Union.
With first-generation vaccine producers already focused on booster doses to take on variants affecting high-income countries, will they be as focused when a variant spreads through lower-income country markets that largely remain unvaccinated? Two-thirds of the WTO’s members support the TRIPS waiver because they are unsure that their COVID-19 public health needs will be met by upholding patent protections as usual. Perhaps this reflects the experience of these countries’ governments in securing access to Pfizer/BioNTech vaccines, let alone the building blocks of knowledge to develop second-generation vaccines. Pfizer has been asking governments to put sovereign assets—such as federal bank reserves, embassy buildings, or military bases—as a guarantee against indemnifying the cost of future legal cases.
What else, beyond resolving intellectual property issues, is necessary to scale up vaccine production?
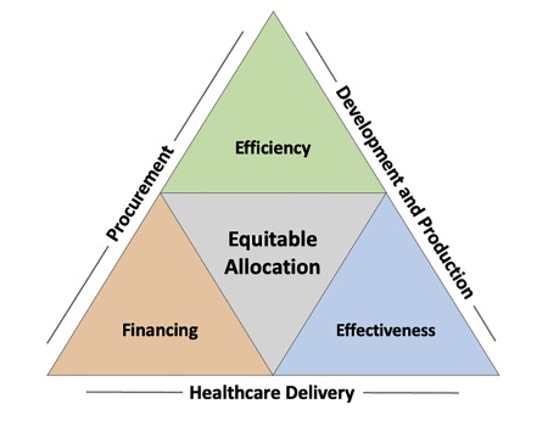
These negotiations over a TRIPS waiver will not take place overnight, nor will scaling up the technology transfer and ramping up the manufacture of COVID-19 vaccines. In a perspective piece for Cell’s new translational science journal, we recently discussed the complex issues involved in scaling up vaccine production and, importantly, ensuring that these vaccines make their way not only to high-income countries, but also more equitably to those in need across the globe. This will involve, among other things:
- Public investment in technology transfer
- Contracting of existing and new manufacturing facilities
- Sourcing other inputs like glass vials
- Pooled procurement facilities, from UNICEF to the Pan American Health Organization’s Revolving Fund for Vaccine Access, to buy and deliver the vaccines effectively.
We will also need to prioritize scaling up second-generation vaccines that have been adapted to address emerging variants or that are better suited for delivery where ultra-cold chains do not exist.
Source: So AD, Woo J. Achieving path-dependent equity for global COVID-19 vaccine allocation. Med 2021; 2(4): P373-377. DOI: https://doi.org/10.1016/j.medj.2021.03.004
What are the next steps?
For years to come, we will need COVID-19 vaccines. A sustainable and affordable pipeline—one that will deliver in a timely way to all in need, not just to those in wealthy countries—must be put in place.
In the best-case scenario, a TRIPS waiver for sharing COVID-19-related knowledge and technology can lay an important foundation to an innovation ecosystem that ensures a fairer path out of the pandemic than we took going into the pandemic.
But no one doubts that there is a tough road ahead in negotiating the text to this waiver and in all the work that follows so that it might make an effective difference. Other questions will naturally arise, including the approach to intellectual property for tests and treatments for COVID-19, as well as the sharing of key research findings and data.
In the near term, the U.S. should also commit to sharing-and-exchange mechanisms for COVID-19 vaccines, especially as uptake of vaccines slows.
We had proposed a temporal trade, for example, on the U.S.-reserved doses of the not-yet-approved AstraZeneca/Oxford vaccine with countries waiting and ready to use this vaccine now. By just swapping our line in the queue, vaccine doses may reach those in desperate need sooner. More such arrangements will be needed, particularly since AstraZeneca/Oxford vaccine supplies globally have since been disrupted with the unfolding COVID-19 resurgence in India, from where much of its manufacture had been sourced.
In the meantime, the world must not lose any time in scaling up the public sector investment in the rest of the supply chain, from manufacturing to logistical delivery of these vaccines. The U.S. can lead a coalition of the willing to build upon and extend the use of such vaccine platforms through technology transfer. History will remember what is done to meet this moment.
Anthony So, MD, MPA , is a professor of the practice in International Health and the founding director of the Innovation+Design Enabling Access (IDEA) Initiative .
RELATED CONTENT
- The New Technology Behind COVID-19 RNA Vaccines and What This Means for Future Outbreaks
- Can COVID-19 Vaccines Be Mandatory in the U.S. and Who Decides?
- Enhancing Public Trust and Health with COVID-19 Vaccination

Related Content

Outbreak Preparedness for All

What’s Happening With Dairy Cows and Bird Flu

New Grant Enables Johns Hopkins Researchers to Implement Community Health Worker-Led Health Interventions for Noncommunicable Diseases in Nepal

How Dangerous is Dengue?
New Johns Hopkins Institute Aims to Safeguard Human Health on a Rapidly Changing Planet
The June 17, 2022 WTO Ministerial Decision on the TRIPS Agreement
Updated Friday, 17 June 2022, 11:30 AM Geneva time.
Early Friday morning, 17 June 2022, the WTO’s 12th Ministerial Conference (chaired by Timur Suleymenov, Kazakshstan), adopted a Ministerial Decision on the TRIPS Agreement (WT/MIN(22)/W/15/Rev.2). The text can be found here .
There were several changes since the June 10 version. The agreement is a limited and disappointing outcome overall that is most accurately described as a narrow and temporary exception to an export restriction, not a waiver.
The Clarifications
There are several so called clarifications in the text on topics, which restate the existing flexibility in TRIPS, sometimes at the risk here of making the provisions seem exceptional, although some developing countries have welcomed them. Among the clarifications, none of which add new legal benefits, are paragraphs 2, 3(a) and 4 of the agreement. The Clarification offered on Article 39.3 of the TRIPS is somewhat helpful, but essentially restates the existing safeguard already part of 39.3. The clarification on paragraph 3(d) and footnote 4 on remuneration do not change TRIPS standards, but will be helpful in national settings, including by the fact that the WTO Ministerial Decision is citing “the Remuneration Guidelines for Non-Voluntary Use of a Patent on Medical Technologies published by the WHO (WHO/TCM/2005.1).” (I am the author of that document).
The TRIPS restriction on exports under Article 31
The TRIPS agreement contains 73 Articles describing various obligations on WTO members as regards the granting and enforcement of intellectual property rights.The original waiver proposal would have provided a clean waiver of 40 Articles in the TRIPS, as regards the manufacturing and supply of any COVID 19 countermeasure. The new considerably scaled back agreement focuses on just one part of the agreement, the 20 word paragraph 31.f which limits exports made under a non-voluntary authorization, often referred to as a compulsory license. The text reads.
(f) any such use shall be authorized predominantly for the supply of the domestic market of the Member authorizing such use;
Article 31.f of the TRIPS is controversial, and often considered an embarrassment to the WTO, because it is designed to limit the economies of scale for products manufactured under a compulsory license, and it also has a very differential impact on countries depending upon their size. For big economies like the U.S., China, India or to some extent Brazil, the impact is less severe than would be for a country like Chile, Ecuador, Portugal, New Zealand, or Thailand, but for every country, it is an odd provision for an organization created to liberalize trade and exploit comparative advantages.
The original TRIPS agreement contains several important workarounds from the 31.f restrictions on exports. Most obviously, Article 31.k of TRIPS waives 31.f, when a compulsory license is a remedy to an anti-competitive practice, which can include, among other grounds, a finding that prices are excessive, or that a patent holder refuses to license a technology on reasonable terms, or that the patented invention is an essential facility, all highly relevant grounds for a vaccine compulsory license.
There is also the possibility to export under Article 30 of the TRIPS, if an exception passes a three step test. The Article 30 approach was strongly supported by health NGOs, generic manufacturers, the World Health Organization (WHO), and several countries in the 2002.2003 negotiations over this issue, and even tested in a 2000 WTO TRIPS dispute case ( DS114 ) relating to the early working of patented inventions, but opposed by the European Union, which favored what is now Article 31bis of the TRIPS.
Arguably the best way to address the export issue is found in the enforcement section of the TRIPS. Under Article 44 of TRIPS, a government or a judicial authority can limit the remedies to infringement to the payment of royalties, including cases where 100 percent of manufacturing output is exported.
In terms of state practice, the Articles 31.k and 44 approaches are the most widely used, by far. (More on the export alternatives here .)
Article 31bis
Article 31bis of the TRIPS was initially adopted by the WTO General Council on August 30, 2003, as an optional waiver of Article 31.f. The core elements of 31 bis are notifications to the WTO, anti-diversion measures, restrictions on eligibility and scope.
31bis applies to drugs, vaccines and some diagnostic tests. It is permanent. It applies to all diseases. 31bis limits the members that can benefit as importers.
In the 19 years since its adoption, the 31bis mechanism has been successfully used only once, by Apotex in Canada for an export of an HIV drug to Rwanda, working with MSF. Apotex indicated it would never attempt to use the mechanism again, giving the complexity and delays they experienced. The Article 31bis mechanism is implemented through national laws, and much of the problems Apotex faced were due to Canadian law and the Canadian government administration of the statute. Few countries have bothered to implement Article 31bis in national statutes, but among those that have, the statutes in India and China offer far more streamlined approaches. India’s version is Section 92a of the patents act, titled “Compulsory licence for export of patented pharmaceutical products in certain exceptional circumstances.” The first two paragraphs in Section 92A of the India patents act read as follows:
(1) Compulsory licence shall be available for manufacture and export of patented pharmaceutical products to any country having insufficient or no manufacturing capacity in the pharmaceutical sector for the concerned product to address public health problems, provided compulsory licence has been granted by such country or such country has, by notification or otherwise, allowed importation of the patented pharmaceutical products from India. (2) The Controller shall, on receipt of an application in the prescribed manner, grant a compulsory licence solely for manufacture and export of the concerned pharmaceutical product to such country under such terms and conditions as may be specified and published by him.
To date, the India statute has not been tested, but pending compulsory licenses for COVID therapeutics in Latin America may provide timely tests, if any of the cases succeed in the Latin American county.
It is important to note that Article 31bis of the TRIPS is very long, including an Article 31bis, an “Annex to the TRIPS” and and “Appendix to the Annex to the TRIPS Agreement.” The WTO analytical index for 31bis is more than five pages single spaced.
Article 31bis notifications
The Article 31bis notifications requirements are seen as a significant problem, in part because governments have to make notifications to the WTO before the exports take place, and also that the notifications involve quantities, even in cases where the importing country is not certainly how many units to purchase, or when the government is not the sole market for the product.
Governments around the world often see the use of compulsory licensing of patented inventions as politically sensitive, inviting considerable pressure from the United States, the European Union and several of its members and Switzerland. WTO notifications on the prior use of a compulsory license will typically involve several ministers or agency heads, including those working on trade, foreign affairs, health and intellectual property rights, if not heads of state. 31bis requires such notifications from both importing and exporting countries. By, as a practical matter, involving trade and foreign affairs officials, there is a greater opportunity for bilateral pressures to block actions. KEI has worked on multiple cases where importing countries were not willing to make a notification as potential importers without having a committed supplier, and the suppliers could not get their governments to make a notification regarding exporting, without the importing country having made its notifications. All of this has to be repeated for each authorization, which includes specifications of qualities and designations. These were the Rwanda and Bolivia notifications as importers, the only two received by the WTO in 19 years. ( Bolivia , Rwanda ), and Canada as an exporter, the one time an export was actually approved under the 31bis mechanism. ( Canada )
Article 31bis anti-diversion obligations Article 31bis does contain several sections regarding the obligations on importing and exporting countries to prevent the re-exportation of the products. Governments are not sure how burdensome the obligations are in practice, but they do go beyond the obligations included in the pre-31bis TRIPS text, and seem to be conflict with Articles 6 of the TRIPS on the exhaustion of rights. They may also increase the costs to the supplier if they cannot re-export unused products when the forecast demand is not met in one country.
The new Ministerial Decision on the TRIPS Agreement
The new Ministerial Decision on the TRIPS Agreement provides an exception to 31.f export restrictions that is temporary, applies only to vaccines and only to COVID 19, limits which countries and import or export, and contains notification and anti-diversion oblations.
Eligibility for importers and exporters
As noted, Article 31bis provides no limits on which countries can use the exception as exporters, but does limit the eligible importing countries. The 31bis limits on importing eligibility is implemented through an opt-out process, and countries were allowed to opt out as importers in general (37 members have done so, see open letter of April 7, 2020 ), or to declare they would only use the mechanism in emergencies.
The new Ministerial Decision on TRIPS defines eligibility for both importing and exporting in footnote 1:
For the purpose of this Decision, all developing country Members are eligible Members. Developing country Members with existing capacity to manufacture COVID-19 vaccines are encouraged to make a binding commitment not to avail themselves of this Decision. Such binding commitments include statements made by eligible Members to the General Council, such as those made at the General Council meeting on 10 May 2022, and will be recorded by the Council for TRIPS and will be compiled and published publicly on the WTO website.
While the opt-out language on eligibility is similar to the approach taken in the August 30, 2003 waiver of Article 31.f of the TRIPS, which is now part of TRIPS as 31 bis , there is an important difference. The new agreement limits the countries eligible to import or export.
There is an expectation that all non-developing countries with the current ability to manufacture and export vaccines will opt out. The status of China seems to be addressed in the reference to the May 10 meeting of the General Council, where China expressed willingness to opt out. This is in some ways an astonishing result. The WTO will continue to enforce export restrictions on vaccines from most of the leading vaccine manufacturing countries including many with sophisticated technology. At an NGO briefing during the negotiations, WTO DG Ngozi Okonjo-Iweala seemed to justify this outcome on the grounds that it would be desirable protectionism to achieve the objective of promoting vaccine manufacturing capacity in Africa and other developing countries.
Notifications
The new Decision had marginally less problematic notifications, the most notable improvement is that notifications can be made “as soon as possible after the information is available,” and while it is not exactly clear how different the requirement is, it does seem like an improvement from 31bis.
Anti-diversion
On the anti-diversion language, the new Decision has one notable but perhaps narrow improvement over 31bis, stating that “in exceptional circumstances, an eligible Member may re-export COVID-19 vaccines to another eligible Member for humanitarian and not-for-profit purposes, as long as the eligible Member communicates in accordance with paragraph 5.” NGOs such as TWN have pointed of that the Ministerial Decisions begins by “Noting the exceptional circumstances of the COVID-19 pandemic,” so hard to say how to interpret the “in exceptional circumstances” language here.
The time period is 5 years, which severely limits its usefulness. Paragraph 5 states:
An eligible Member may apply the provisions of this Decision until 5 years from the date of this Decision. The General Council may extend such a period taking into consideration the exceptional circumstances of the COVID-19 pandemic. The General Council will review annually the operation of this Decision.
The exception to the export restrictions will only last 5 years. There are currently no developing country vaccines manufactured under a compulsory license. Moderna is operating under a compulsory license from the United States, but the US will not be an eligible exporter. For the Ministerial Decision to have any use for vaccines, a developing country would have to issue a compulsory license on a vaccine or vaccine input, obtain regulatory approval for that vaccine, and export more than 50 percent of output. Under optimistic scenarios, it could take 2 to 3 years to bring a new COVID vaccine into the market, given the increasing challenges in obtaining regulatory approval not that emergency use authorizations have already been used for multiple vaccines. And that would only give the developer, if they began work today, a few years of sales under the exception. For this reason alone, the Indian government has predicted the Ministerial Decision would not lead to any new vaccine manufacturing. ( June 14, 2022, Statement by Shri Piyush Goyal during the WTO 12th Ministerial Conference at the meeting with co-sponsors of TRIPS Waiver )
“Second, with great difficulty we got the period of 5 years. But, we all know that by the time we get an investor, get funds raised, draw plans, get equipment and set up a plant, it will probably take 2.5-3 years to do that. After that, you will start producing and within 2 years, you will have to bring down your exports to the normal compulsory license level and your capacity will remain idle.”
The Ministerial Decision text will be tied with 31 bis as one of the worst ways to allow exports under a compulsory license. Articles 31.k, 30 and 40 all will dominate. (More on the alternatives here ).
The big pharma industry can be pleased with the precedents on notifications and anti-diversion, which are important to them, as well as the exclusion of most vaccine manufacturers and the 5 year duration.
It is hard to imagine anything with fewer benefits than this, as a response to a global health emergency (other than the earlier negotiating texts for this Decision). The fact that the exception is limited to vaccines, has a five year duration and does not address WTO rules on trade secrets makes it particularly unlikely to provide expanded access to COVID 19 countermeasures.
The pressure this week was to reach consensus in order to make multilateralism look like it works, which seems to have been the main justification for producing this decision.
Silver linings While the text is not expected to impact COVID 19 vaccine equity much or at all, there are some silver linings.
1. In terms of precedents going forward, the texts of notifications and anti-diversion are both much shorter and more usable than 31bis. 2. There is no 31bis requirement to limit imports to countries with no or insufficient manufacturing capacity, a sometimes ambiguous standard oddly unconnected to economic feasibility. 3. The fact that the Decision cites “the Remuneration Guidelines for Non-Voluntary Use of a Patent on Medical Technologies published by the WHO (WHO/TCM/2005.1)” will be useful in national settings. 4. If the decision is extended to therapeutics in six months, it may be much more value, given the supply constraints on therapeutics and the much better regulatory pathway. For therapeutics, even the language on 39.3 will be useful for some countries. 5. It’s not a TRIPS waiver, it’s a useful edit of some problematic elements of 31bis. But it also turns attention back to national governments to do things, and not wait on the WTO.
James Love Twitter: @jamie_love
- Access to Knowledge
- Access to Medicine
- Competition
- Government Funded research
- Intellectual Property Rights
- Legislation
- Negotiations
- Nuclear Proliferation
- Orphan Drugs
- Presentations
- Public Goods
- Research and Development
- Site Articles
- Transparency

- WHAT THEY ARE SAYING: Doug McKalip Confirmed as USTR Chief Agricultural Negotiator
- Statement from Ambassador Katherine Tai Following Doug McKalip’s Senate Confirmation Vote
- Statement from USTR Spokesperson Adam Hodge
- USTR Announces Additional Senior Staff Members
- United States Requests New USMCA Dispute Consultations on Canadian Dairy Tariff-Rate Quota Policies
- Readout of Ambassador Jayme White’s Meeting with Mexico’s Under Secretary of Economy Alejandro Encinas
- Ambassador Tai Requests USITC Investigation of COVID-19 Diagnostics and Therapeutics
- Joint Statement from Ambassador Tai and Secretary Vilsack after Meeting with Mexican Government Officials
- USTR Extends Exclusions from China Section 301 Tariffs
- Joint USTR and Department of Commerce Readout of the First Indo-Pacific Economic Framework Negotiating Round
- Statement by Ambassador Katherine Tai on the Signing of the Memorandum of Understanding on Cooperation for Trade and Investment Between the United States and the African Continental Free Trade Area
- Readout of Ambassador Katherine Tai and Ambassador Sarah Bianchi’s Events on Day Two of the U.S. – Africa Leaders Summit
- Readout of the African Growth and Opportunity Act Ministerial During the U.S.-Africa Leaders Summit
- Readout of Ambassador Katherine Tai’s Meeting With Kenya’s Ministry of Investments, Trade and Industry, Cabinet Secretary Moses Kuria
- What They Are Saying: Ambassador Katherine Tai Confirms Amendments to the Beef Safeguard Trigger Level Under the U.S.-Japan Trade Agreement
- Protocol Amending the Beef Safeguard Provisions of the U.S.-Japan Trade Agreement to Enter into Force on January 1, 2023
- Readout of Ambassador Katherine Tai's Meeting with Canada's Minister of Labor Seamus O'Regan
- United States and Bangladesh Convene 6th Meeting of the U.S.-Bangladesh Trade and Investment Cooperation Forum Agreement Council
- Joint Statement on the Third Meeting of the United States – Argentina Council on Trade and Investment
- United States and Panama Hold the Third Meeting of the Environmental Affairs Council under the United States-Panama Trade Promotion Agreement
U.S. to Support Extension of Deadline on WTO TRIPS Ministerial Decision; Requests USITC Investigation to Provide More Data on COVID-19 Diagnostics and Therapeutics
- U.S.-EU Joint Statement of the Trade and Technology Council
- USTR, Department of Labor, European Commission Host Inaugural Principals’ Meeting of the U.S.-EU Trade and Labor Dialogue with Union, Business Leaders
- United States and European Union Conclude First Ministerial Meeting of the Large Civil Aircraft Working Group
- United States and Peru Hold Meetings of the Environmental Affairs Council and the Sub-Committee on Forest Sector Governance under the United States-Peru Trade Promotion Agreement
- Readout of Ambassador Katherine Tai's Meeting with Mexico’s Secretary of Economy Raquel Buenrostro
- PRESS ADVISORY: Media Registration for Third U.S.-EU Trade and Technology Council Ministerial Meeting
- Policy Offices
- Press Office
- Press Releases
December 06, 2022
USTR releases summary of five-month consultation on extending WTO TRIPS decision showing broad divergence of views
WASHINGTON – The Office of the United States Trade Representative today announced support for extending the deadline to decide whether there should be an extension of the World Trade Organization (WTO) Ministerial Decision on the TRIPS Agreement (Ministerial Decision) to cover the production and supply of COVID-19 diagnostics and therapeutics. USTR also announced that it will ask the United States International Trade Commission (USITC) to launch an investigation into COVID-19 diagnostics and therapeutics and provide information on market dynamics to help inform the discussion around supply and demand, price points, the relationship between testing and treating, and production and access.
“Over the past five months, USTR officials held robust and constructive consultations with Congress, government experts, a wide range of stakeholders, multilateral institutions, and WTO Members,” said Ambassador Katherine Tai. “Real questions remain on a range of issues, and the additional time, coupled with information from the USITC, will help the world make a more informed decision on whether extending the Ministerial Decision to COVID-19 therapeutics and diagnostics would result in increased access to those products. Transparency is critical and USTR will continue to consult with Congress, stakeholders, and others as we continue working to end the pandemic and support the global economic recovery.”
Supporters and opponents of extending the Ministerial Decision to COVID-19 diagnostics and therapeutics provided extensive views and arguments. USTR officials also reviewed and analyzed published information, opinions, and analysis. In both cases, the views concern both the system as a whole – whether existing WTO intellectual property protections are an impediment to access to medicines or a critical element of innovation – as well as the specific characteristics of the markets for COVID-19 diagnostics and therapeutics.
The United States respects the right of its trading partners to exercise the full range of existing flexibilities in the TRIPS Agreement, such as in Articles 30, 31, and 31 bis , and the Doha Declaration on the TRIPS Agreement and Public Health, as well as the flexibilities in the Ministerial Decision. These existing flexibilities are available as part of the effort to scale up the production and distribution necessary to overcome the challenges of the ongoing COVID-19 pandemic.
Based on available data and public input, the USITC study will explore key issues such as:
- An overview of the products, focusing on WHO-approved COVID-19 diagnostics and therapeutics, including key components, the production process, intellectual property protections, and a description of the supply chain (including the level of diversification in the supply chain);
- Information on the global manufacturing industry for these products, including information on key producing countries, major firms, and production data, if available;
- Information on the global market for COVID-19 diagnostics and therapeutics, including information on demand and, to the extent practicable, an assessment of where unmet demand exists for key products and contributing factors; market segmentation; and supply accumulation and distribution;
- Data and information on global trade in COVID-19 diagnostics and therapeutics, if available, or if not, data and information on global trade in diagnostics and therapeutics generally; and
- A brief overview/background of the relevant aspects of the TRIPS Agreement and the United Nations (UN) Medicine Patent Pool (MPP) and a listing of countries seeking to use the Ministerial Decision and those utilizing access to COVID-19 medicines under the MPP.
As part of the Administration’s comprehensive effort to combat the pandemic, the United States supported negotiations that resulted in the WTO issuing the Ministerial Decision on the TRIPS Agreement on June 17, 2022. Since then, USTR officials consulted with Members of Congress and more than two dozen stakeholders, including public health advocates, organized labor, academics, think tanks, companies, and trade associations. USTR has summarized the diverse views heard during the five-month consultation period.

- 600 17th Street NW
- Washington, DC 20508

- Reports and Publications
- Fact Sheets
- Speeches and Remarks
- Blog and Op-Eds
- The White House Plan to Beat COVID-19
- Free Trade Agreements
- Organization
- Advisory Committees
- USTR.gov/open
- Privacy & Legal
- FOIA & Privacy Act
- Attorney Jobs
Programs submenu
Regions submenu, topics submenu, the role of fast payment systems in addressing financial inclusion, modernizing army software acquisition: panel discussion with dasa(sar) margaret boatner and peo iew&s bg ed barker, book event: the mountains are high, energy security and geopolitics conference.
- Abshire-Inamori Leadership Academy
- Aerospace Security Project
- Africa Program
- Americas Program
- Arleigh A. Burke Chair in Strategy
- Asia Maritime Transparency Initiative
- Asia Program
- Australia Chair
- Brzezinski Chair in Global Security and Geostrategy
- Brzezinski Institute on Geostrategy
- Chair in U.S.-India Policy Studies
- China Power Project
- Chinese Business and Economics
- Defending Democratic Institutions
- Defense-Industrial Initiatives Group
- Defense 360
- Defense Budget Analysis
- Diversity and Leadership in International Affairs Project
- Economics Program
- Emeritus Chair in Strategy
- Energy Security and Climate Change Program
- Europe, Russia, and Eurasia Program
- Freeman Chair in China Studies
- Futures Lab
- Geoeconomic Council of Advisers
- Global Food and Water Security Program
- Global Health Policy Center
- Hess Center for New Frontiers
- Human Rights Initiative
- Humanitarian Agenda
- Intelligence, National Security, and Technology Program
- International Security Program
- Japan Chair
- Kissinger Chair
- Korea Chair
- Langone Chair in American Leadership
- Middle East Program
- Missile Defense Project
- Project on Critical Minerals Security
- Project on Fragility and Mobility
- Project on Nuclear Issues
- Project on Prosperity and Development
- Project on Trade and Technology
- Renewing American Innovation Project
- Scholl Chair in International Business
- Smart Women, Smart Power
- Southeast Asia Program
- Stephenson Ocean Security Project
- Strategic Technologies Program
- Transnational Threats Project
- Wadhwani Center for AI and Advanced Technologies
- All Regions
- Australia, New Zealand & Pacific
- Middle East
- Russia and Eurasia
- American Innovation
- Civic Education
- Climate Change
- Cybersecurity
- Defense Budget and Acquisition
- Defense and Security
- Energy and Sustainability
- Food Security
- Gender and International Security
- Geopolitics
- Global Health
- Human Rights
- Humanitarian Assistance
- Intelligence
- International Development
- Maritime Issues and Oceans
- Missile Defense
- Nuclear Issues
- Transnational Threats
- Water Security
TRIPS Waivers and Pharmaceutical Innovation

Blog Post by Chris Borges
Published March 15, 2023
By Christopher Borges
On June 22, 2022, the World Trade Organization (WTO) approved a waiver of intellectual property (IP) protections for COVID-19 vaccine patents, previously secured under the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS). The WTO is currently considering expanding this waiver to include COVID-19 diagnostics and therapeutics in addition to vaccines. As the U.S. International Trade Commission (USITC) investigates the implications of this expansion, it is important to understand what this waiver is intended to accomplish, explore whether it will be effective in the short term, and examine the long run impacts on the bio-pharmaceutical innovation system.
What Is the TRIPS Agreement?
TRIPS refers to a WTO agreement incorporating obligations related to IP protection into the global rules-based trading system. Active since 1995, TRIPS requires most WTO members to adhere to minimum rules for the protection of IP — such as patents, copyrights, and trademarks — and enforce these commitments domestically. By agreeing to respect IP protections, member countries receive certain benefits in return. For example, TRIPS allows WTO members to make exceptions to patent rights so long as they are “limited” and do not violate the “normal” use of the patent. States often employ this provision to advance their science and technology base by allowing their researchers to use patented research tools and techniques.
Why Was There a Call to Waive TRIPS IP Protections for COVID-19 Vaccines?
Despite COVID-19 vaccines being the fastest developed vaccines in history, global access to these vaccines remains uneven. The United States first administered COVID-19 vaccines in December 2020, yet, per the University of Oxford, as of March 1st, 2023, only 28 percent of people in low-income countries have received at least one dose of a COVID-19 vaccine.
Most of the vaccines approved for use are developed by firms in the United States, Europe, China, and Russia, but the Western-made mRNA vaccines are the most effective and therefore the most in-demand vaccines on the market. The wealth of Western nations along with the geographic distribution of mRNA vaccine producers enabled them to reserve large vaccine supplies early in the pandemic, effectively shutting out lower-income countries. Low-income countries currently have a 28 percent vaccination rate, whereas the United States had vaccinated 28 percent of its population by March 23rd, 2021.
Citing this disparity, many developing nations called on the international community to waive TRIPS IP protections for COVID-19 vaccines, based on the notion that allowing any company to manufacture the vaccines will boost production and, ultimately, vaccinations. South Africa and India first proposed a TRIPS waiver for COVID-19 vaccines in October 2020, drawing considerable support from over 100 lower-income countries. High-income countries, however, were initially opposed to the waiver on the grounds that it would have an adverse effect on innovation, drug quality, and drug safety. Negotiations continued for nearly two-years until the waiver was ultimately agreed to, with high-income countries easing their objections once they were sufficiently supplied with vaccines.
What Does the COVID-19 Vaccine Waiver Do?
The COVID-19 vaccine waiver suspends certain requirements regarding the use of COVID-19 vaccine patents, such as ingredients and manufacturing processes. With this waiver, states can authorize domestic manufacturers to produce COVID-19 vaccines without the permission of the patent rights holder and, crucially, to export those vaccines to other countries.
The waiver was designed to be a short term action, taken as an emergency measure in the midst of a global pandemic. However, as implementing the waiver required all 164 WTO members to agree, it took nearly two years of deliberation to come to consensus. By the time WTO members agreed to the waiver in June 2022, the response to the pandemic had progressed considerably and over 12 billion vaccine doses had been administered.
Why Are There Calls to Expand the TRIPS Waiver?
The WTO is currently considering if the waiver should be expanded to include the production and supply of COVID-19 diagnostics and therapeutics. This would cover a broad category of products, including products utilized for diseases and conditions beyond COVID-19.
To date, the FDA has approved dozens of COVID-19 therapeutics. Oral antiviral treatments paxlovid and molnupiravir were quickly developed by Pfizer and Merck, respectively, and approved by the FDA in late 2021. Remsidivir, a therapeutic first developed in 2009 by Gilead Sciences, was repurposed to treat COVID-19 after studies concluded that it reduces the risk of hospitalization and death in high-risk patients by up to 87 percent.
The efficacy of these COVID-19 treatments prompted low-income countries and international organizations such as UNICEF to demand an expansion of the TRIPS waiver to include these drugs along with diagnostic tests. To continue combating COVID-19 — the World Health Organization (WHO) reported 70,000 new cases on March 1st — they assert that all medical tools must be made available to the fullest extent.
How Effective Is the TRIPS Waiver? How Effective Would the Expansion Be?
The COVID-19 TRIPS waiver has had minimal impact on overall vaccine access. As of the end of 2022, no country had declared intent to make use of the TRIPS waiver.
Global vaccine demand had plummeted by the time the TRIPS waiver was agreed to. In December 2022, the board of Gavi, a nonprofit that supplies vaccines to low- and middle-income countries voted to stop supplying COVID-19 vaccines to most nations due to lack of demand. This drop in demand indicates that the primary issue impeding vaccinations today is not lack of supply, but lack of distribution capacity. Administering COVID-19 vaccines across a population requires significant healthcare infrastructure which some developing countries lack , such as refrigeration to keep vaccines at low temperatures and a well-trained healthcare workforce. To increase global vaccination rates, efforts should focus on building healthcare infrastructure and distribution capacity, not facilitating additional vaccine production.
Currently, the supply of treatments to COVID-19 far outstrips demand as well. This is largely because secure IP rights have incentivized drug inventors to enter over 140 partnerships with manufacturers worldwide, boosting supply while transferring technology and tacit knowledge to these foreign firms. Secure IP rights assure companies that their inventions will not be stolen in the short-term, thereby allowing them to reveal their secrets and participate in these productive manufacturing partnerships. Expanding the TRIPS waiver to therapeutics would have little added benefit to access.
Further, expanding the TRIPS waiver to therapeutics will disincentivize the creation of new COVID-19 treatments. Biopharmaceutical research is expensive and risky — the R&D process for new drugs costs close to $1 billion on average, and only 12 percent of drugs which enter clinical trials are ultimately approved for use. Companies will simply not invest in creating new therapeutics if they will lose ownership of their IP should their huge and risky investment prove fruitful.
Can the COVID-19 TRIPS Waivers Damage the Biopharma Innovation Ecosystem?
IP rights advocates point out that undermining IP protections will weaken incentives for pharmaceutical companies to innovate. Bio-pharmaceutical research and development (R&D) costs are so high that private capital will not invest without the promise of exclusive rights on the output. While quick government action and spending in the early days of the pandemic accelerated the development of COVID-19 vaccines, the rapid response to COVID-19 was built on the long-term stability of IP protections.
For example, the science and technology behind mRNA vaccines, an essential tool in the fight against COVID-19, was supported over decades by both far-sighted government investment as well as through commercialization drawing on considerable private capital expecting a return. The success of mRNA vaccines was not a slam dunk, yet investors took the risk on the understanding that they would receive substantial returns should the technology prove effective. Throughout this long and risky R&D process, the secure and predictable assignment of property rights allowed universities, government labs, and large and small companies to cooperate effectively to develop mRNA vaccine technology, and, ultimately, deliver vaccines in record time.
By removing IP protections on COVID-19 vaccines and treatments, the WTO is weakening the incentives for companies to invest in financially risky technology in the future as, even if their venture is successful, they may lose IP protections which allow them to recoup their investment.
How Will the TRIPS Waivers Impact U.S. National Security?
Global trends such as climate change, urbanization, and rising meat consumption make future pandemics more likely. It is critical that the United States maintain a dynamic and innovative pharmaceutical industry to combat this threat.
Government action, in partnership with private industry, in the early days of the pandemic accelerated the rapid development and scale up of COVID-19 vaccines. Through a myriad of policies such as pre-ordering millions of vaccine doses, Operation Warp Speed expedited the development and roll-out of vaccines by months, saving thousands of lives.
While quick action played a key role in the overall response, however, a crucial lesson from COVID-19 is that waiting until a pandemic is declared to act will be too late. mRNA vaccine technology was developed over decades and sustained by a dynamic and innovative bio-pharmaceutical ecosystem that connects universities, government labs, and large and small firms in the industry. Because of this large body of pre-existing work, much of which was facilitated through the security and predictability afforded by IP protections, pharmaceutical companies were able to prototype COVID-19 vaccines within days of receiving the viral genome. Further, this ecosystem not only rapidly produced dozens of COVID-19 therapeutics, but also possessed an existing supply of drugs that proved effective in treating COVID-19. Without a long-standing healthy innovation ecosystem, this could not have happened.
TRIPS waivers undermine the U.S. pharmaceutical industry by degrading the IP protections which are essential to the pharmaceutical innovation ecosystem. Less innovation in the pharmaceutical industry means fewer vaccines and drugs in the future, leaving the United States and other nations less prepared for future pandemics and other health emergencies.
Christopher Borges is a research intern with the Renewing American Innovation project at the Center for Strategic and International Studies in Washington, D.C.
The Perspectives on Innovation Blog is produced by the Renewing American Innovation Project at the Center for Strategic and International Studies (CSIS), a private, tax-exempt institution focusing on international public policy issues. Its research is nonpartisan and nonproprietary. CSIS does not take specific policy positions. Accordingly, all views, positions, and conclusions expressed in this publication should be understood to be solely those of the author(s).
© 2024 by the Center for Strategic and International Studies. All rights reserved.

Chris Borges
Programs & projects.
- Standards and Intellectual Property Rights for Innovation
- Strengthening the U.S. Innovation Ecosystem

Improving Access to COVID-19 Vaccines: An Analysis of TRIPS Waiver Discourse among WTO Members, Civil Society Organizations, and Pharmaceutical Industry Stakeholders
Volume 24/2, December 2022, pp. 159-175 | PDF
Jillian Kohler, Anna Wong, and Lauren Tailor
Throughout the COVID-19 pandemic, international access to COVID-19 vaccines and other health technologies has remained highly asymmetric. This inequity has had a particularly deleterious impact on low- and middle-income countries, engaging concerns about the human rights to health and to the equal enjoyment of the benefits of scientific progress enshrined under articles 12 and 15 of the International Covenant on Economic, Social and Cultural Rights. In response, the relationship between intellectual property rights and public health has reemerged as a subject of global interest. In October 2020, a wholesale waiver of the copyright, patent, industrial design, and undisclosed information sections of the Agreement on Trade-Related Aspects of Intellectual Property (TRIPS Agreement) was proposed by India and South Africa as a legal mechanism to increase access to affordable COVID-19 medical products. Here, we identify and evaluate the TRIPS waiver positions of World Trade Organization (WTO) members and other key stakeholders throughout the waiver’s 20-month period of negotiation at the WTO. In doing so, we find that most stakeholders declined to explicitly contextualize the TRIPS waiver within the human right to health and that historical stakeholder divisions on the relationship between intellectual property and access to medicines appear largely unchanged since the early 2000s HIV/AIDS crisis. Given the WTO’s consensus-based decision-making process, this illuminates key challenges faced by policy makers seeking to leverage the international trading system to improve equitable access to health technologies.
Introduction
Article 12 of the 1966 International Covenant on Economic, Social and Cultural Rights (ICESCR) recognizes every person’s human right to the enjoyment of the highest attainable standard of physical and mental health, while article 15 recognizes every person’s human right to enjoy the benefits of scientific progress. [1] Taken together, this necessarily includes every person’s right to access lifesaving health technologies, such as vaccines, pharmaceuticals, personal protective equipment, and diagnostics. Yet inequities persist, with as many as two billion people in low- and middle-income countries (LMICs) lacking regular access to essential medicines. [2]
Throughout the COVID-19 pandemic, global access to COVID-19 vaccines has remained highly asymmetric despite efforts by global institutions, such as COVAX, to advance such access. [3] When combined with general product shortages, price gouging, export restrictions on health supplies, vaccine manufacturing know-how constraints, and “my nation first” procurement approaches by high-income countries, equitable access to COVID-19 diagnostics and health technologies has been severely undermined. [4] This inequity in access to medicines and health technologies has had a particularly deleterious impact on vulnerable groups throughout the pandemic, notably in LMICs. [5] Two years since the start of the pandemic, several high-income countries have achieved full vaccination coverage in 70%–99% of their populations, while only 15.8% of people in low-income countries have received at least a single dose. [6]
Amid this unequal access to essential medicines and health technologies, the relationship between intellectual property rights and public health has reemerged as a subject of global concern. Pursuant to the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement), all World Trade Organization (WTO) members have an obligation to respect patents issued within their domestic intellectual property (IP) systems irrespective of a patented invention’s initial country of origin. [7] This includes all patents that protect technology essential to the manufacture of COVID-19 vaccines.
Patents are a type of intellectual property right that provides inventors with the temporary right to exclude others from making, selling, or importing their patented technology. [8] As such, patents serve to limit supply and raise prices when manufacturers exercise their monopoly power to under-produce needed pharmaceuticals or charge prices that are out of reach for the majority of populations. [9] These issues are not new and have been raised in discussions surrounding the supply of pharmaceuticals for major diseases, including HIV/AIDS and hepatitis C. [10] Given that nearly all COVID-19 vaccines approved for use are protected by at least one active or pending patent, similar concerns have been raised in the production of COVID-19 vaccines and the associated consequences that this has on health equity. [11]
In October 2020, a wholesale waiver of the copyright, patent, industrial design, and undisclosed information sections of the TRIPS Agreement was proposed by India and South Africa at the WTO on the basis that such a measure would be necessary to ensure that intellectual property rights would not interfere with “timely access to affordable medical products … or to [the] scaling-up of research, development, manufacturing and supply of medical products essential to combat[ing] COVID-19.” [12] In May 2021, the waiver was clarified as intended to apply to all COVID-19-related health products and technologies, including vaccines, therapeutics, medical devices, and personal protective equipment. [13] In March 2022, a compromise between the European Union, India, South Africa, and the United States was proposed to narrow the applicability of the waiver to just COVID-19 vaccines. [14] The compromise also sought to limit the availability of the waiver to only those countries that exported less than 10% of the world’s vaccines in 2021. [15] Government responses to the waiver and its proposed alternatives have been divided, but as of June 2022, WTO members agreed to a modified version of the limited March 2022 waiver applicable for five years to COVID-19 vaccines. [16] Further conditions notably include a restriction on the waiver’s availability to only developing country WTO members, country obligations to prevent the re-exportation of products made under the waiver, and a six-month extension of discussions on expanding the waiver’s scope to COVID-19 therapeutics and diagnostics. [17]
To better understand the justifications and implications of the TRIPS waiver negotiations and June 2022 compromise, we identify and evaluate the positions of WTO members and other key stakeholders with respect to the waiver and its relationship to health as a human right. In doing so, we find that historical stakeholder identities and positions with respect to IP and access to medicines have remained largely unchanged. Given the consensus-based decision-making at the WTO, this suggests that political and structural barriers continue to play a large role in limiting policy makers’ ability to leverage the international trading system to improve equitable access to health technologies.
Methodology
A descriptive, qualitative study drawing on critical policy studies methodologies, focusing on how interests, values, and normative assumptions shape and inform policy formation and implementation, was conducted to analyze the public statements of WTO members, pharmaceutical stakeholders, and civil society organizations with respect to the TRIPS waiver. [18] In particular, a combined inductive and deductive thematic analysis was employed to identify reoccurring TRIPS waiver position rationales expressed across each stakeholder and position class and to specifically search for rationales grounded in human rights-related appeals. [19] Data were independently abstracted by AW and LT, with JK resolving any discrepancies through an additional round of review.
Document identification
Official WTO member positions on the TRIPS waiver were sourced from WTO General Council and TRIPS Council meeting minutes from October 2020 to June 2022, as well as all official WTO member submissions related to the COVID-19 TRIPS waiver. [20] Based on these documents, a final list of all WTO members and their positions with respect to the TRIPS waiver was compiled. This list was then verified for consistency with an internal Médecins Sans Frontières policy tracker, which the organization used to construct its public infographic on TRIPS waiver country positions and shared with the authors upon request. [21]
Thirty statements from over 350 civil society organizations included for analysis were extracted from submissions to the WTO’s official COVID-19 public consultation docket and the Canadian Centre for Policy Alternatives TRIPS COVID-19 waiver civil society letter repository (see Appendix 1, available upon request). [22] Sixty-six pharmaceutical companies were included for analysis and were selected based on size (top 20 multinational companies by 2020 revenue) and involvement in COVID-19 product development (COVAX suppliers), with all TRIPS waiver-related press releases recorded. [23] Official statements made by pharmaceutical trade organizations Pharmaceutical Research and Manufacturers of America, Biotechnology Innovation Organization, International Federation of Pharmaceutical Manufacturers and Associations, and European Federation of Pharmaceutical Industries and Associations were also queried (see Appendix 2, available upon request).
Document analysis
Documents were analyzed to identify the following elements: (1) country/organization identity, (2) country/organization position with respect to the TRIPS waiver (support, neutral or undetermined, opposed), and (3) country/organization rationale for their TRIPS waiver position. Stakeholder positions with respect to the TRIPS waiver were determined based on their explicit endorsement or rejection of any iteration of the waiver before the March 2022 compromise, or their qualitative expressions of support for or objection to any iteration of the waiver before the March 2022 compromise. Stakeholders whose positions were expressed in multiple documents were deemed to have adopted the position expressed in the document reporting their most recent public statement. Stakeholders who released statements that did not clearly express support or objection to the TRIPS waiver were classified as neutral or undetermined and, due to the limited public statements available in this category, were not analyzed further for their position rationales. Explicit references to “human rights” or rights-based assertions to health found in these documents were separately extracted for analysis.
Overall, the majority of WTO members and surveyed civil society organizations expressed support for a COVID-19 TRIPS waiver—either in its original October 2020 form or limited March 2022 form. By contrast, nearly all pharmaceutical industry stakeholders who issued public statements voiced opposition to all iterations of the TRIPS waiver. While these positions align strongly with the historical approaches of these stakeholders, a survey of the specific rationales presented by each provides greater insight into the primary sources of axiomatic contention during the COVID-19 TRIPS waiver discussions. The following section provides a breakdown of each of these stakeholder groups’ positions with respect to the waiver, as well as the dominant rationales offered for these positions.
WTO members
Until June 2022, approximately 60% of WTO members expressed support for a TRIPS waiver, either as outright sponsors or through favorable endorsement of the waiver. Approximately 21% of members expressed opposition to the TRIPS waiver (with 28 of the 35 opposing members belonging to the European Union or the European Union delegation itself). The remaining 19% of member positions were undetermined, either because they did not publicly comment on the TRIPS waiver or because their comments refrained from expressing a definitive position with respect to the waiver. The breakdown of WTO member positions and their position rationales is outlined in Table 1.
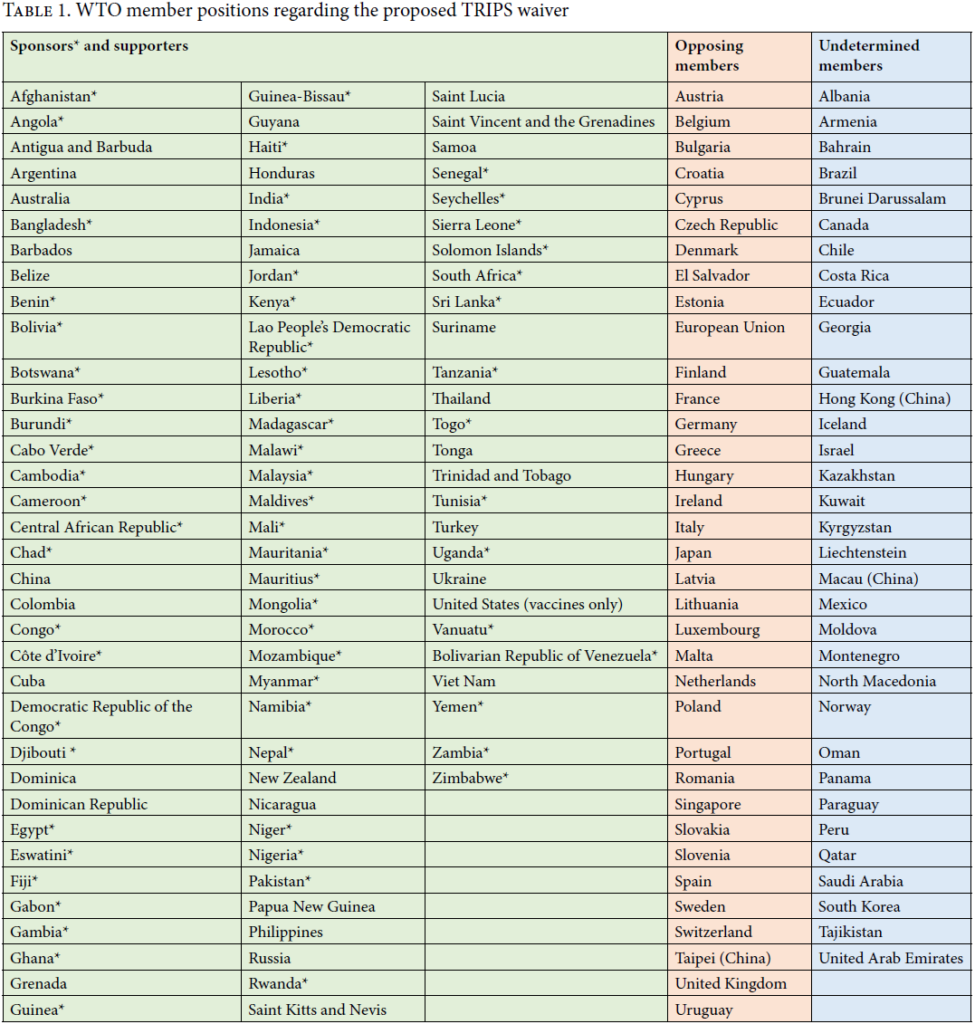
WTO member rationales for supporting a TRIPS waiver. WTO members in support of the TRIPS waiver advanced four main arguments: (1) the TRIPS waiver is required to address IP-based barriers to access that the existing voluntary and compulsory licensing system is ill-equipped to manage; (2) the TRIPS waiver is important as a tool for promoting further COVID-19 solutions that are consistent with the human right to health; (3) the TRIPS waiver should include vaccines, therapeutics, and diagnostics, since a waiver just for vaccines would be insufficient to adequately address COVID-19; and (4) the TRIPS waiver is a legitimate trade policy tool under the existing WTO rules. Below, each rationale is discussed in further detail.
- IP is a barrier to access that cannot be addressed by voluntary or compulsory licensing
Members in support of the TRIPS waiver all adopted the position that IP is actively serving as a supply barrier by preventing the mass manufacture of needed COVID-19 vaccines, therapeutics, and medical devices. Many further argued that this barrier cannot be adequately addressed through voluntary licensing agreements with manufacturers or by issuing compulsory licenses to expand supply. South Africa emphasized that voluntary licensing agreements suffer from a lack of transparency, impose geographic restrictions that often prohibit export even to developing countries, and typically have only a nominal effect on increasing overall market supply. [24] Many countries also argued that the country-by-country and product-by-product approach to compulsory licensing prescribed under articles 31 and 31 bis of the TRIPS Agreement, which enable countries to manufacture and import generic versions of patented products without a patent owner’s consent, undermined the cross-border and widespread use of compulsory licenses required to respond to an international pandemic. India emphasized that ownership disputes among COVID-19 vaccine patent holders would likely compound delays in the articles 31 and 31 bis processes, since countries would potentially need to identify and send notice to multiple litigating owners to ensure compliance with TRIPS compulsory licensing procedures. [25] A lack of domestic legal capacity to engage in compulsory licensing was also highlighted by some states as further support that compulsory licensing is inadequate for supplying COVID-19 vaccines, therapeutics, and medical devices at an international scale. [26]
2. A TRIPS waiver is a tool consistent with promoting the human right to health
Several WTO members highlighted the sharp inequities among high-income and low-income countries regarding access to COVID-19 vaccines. For example, Bangladesh underscored the effects of vaccine nationalism and the lack of access faced by least developed countries, highlighting that the richest 16% of the world had “pre-booked” the majority of vaccines until 2025. [27]
In response to claims by opposing members that COVAX, rather than a TRIPS waiver, was the solution to ensuring equity, supporting members emphasized that “the problem with philanthropy [COVAX] is that it cannot buy equality.” [28] In a position summary document submitted by TRIPS waiver sponsors in September 2021, they underscored that the adoption of the TRIPS waiver would act “as an important political, moral, and economic lever towards encouraging solutions aimed at global equitable access to COVID-19 health products and technologies.” [29] The preambular text of the document emphasized that in seeking equitable health outcomes, the TRIPS waiver was consistent with “the right of everyone to the enjoyment of the highest attainable standard of physical and mental health” protected under article 12 of the ICESCR, as well as the “bold commitment” under United Nations Sustainable Development Goal 3 to ending communicable diseases, achieving universal health coverage, and providing access to safe and effective medicines and vaccines for all. [30]
3. A TRIPS waiver for just vaccines is insufficient
Several supporting members emphasized the importance of including all relevant health technologies—rather than just vaccines—within the scope of the waiver. In particular, sponsors urged WTO members to recall that “vaccines are necessary but not sufficient” and that personal protective equipment, diagnostics, ventilators, and therapeutics are all essential to preventing the spread and ensuring the treatment of COVID-19. [31]
4. A TRIPS waiver is an established and accepted option under existing WTO rules
Many supporting members highlighted that under article IX.3 of the WTO Marrakesh Agreement, waivers of obligations imposed under WTO trade agreements can be legitimately employed in exceptional circumstances. [32] In the October 2020 TRIPS Council meeting, this was affirmed by the WTO Secretariat, which stated that the Ministerial Conference “may decide to waive an obligation imposed on a Member by the Marrakesh Agreement or any of the [WTO’s] multilateral trade agreements.” [33] Supporting members have argued that approving a temporary TRIPS waiver to address urgent public health needs during the COVID-19 pandemic should thus be seen as consistent with, rather than an exception to, the rules-based multilateral trading system. [34]
WTO member rationales for opposing a TRIPS waiver. Four primary rationales were advanced by WTO members opposed to the TRIPS waiver: (1) a TRIPS waiver would not be effective in increasing global supplies since patents and the TRIPS Agreement are not a barrier to access; (2) access to needed COVID-19 health technologies can be addressed through nominal modifications to the TRIPS compulsory licensing system; (3) a TRIPS waiver would introduce legal uncertainty to the international system, thus undermining existing licensing partnerships that are essential to expanding access; and, (4) a TRIPS waiver would undermine the growth of the IP-dependent health technology sector, contrary to domestic development interests. Below, each rationale is discussed in further detail.
- Patents and existing TRIPS obligations are not a barrier to access
Nearly all opposing members endorsed a view that the patent and other IP protection obligations mandated under the TRIPS Agreement are not a primary factor responsible for limiting access to needed COVID-19 vaccines, therapeutics, or medical devices. Instead, members urged that temporary demand shocks, manufacturing capacity constraints, and supply chain delays were “much more likely to have an impact on access than [intellectual property rights].” [35] Several members cited a 2021 interview with the Serum Institute of India’s CEO Adar Poonawalla, who stated that he believed global supply shortages were due to short-term scale-up delays rather than insufficient licensing to generic manufacturers by patent owners. [36]
2. Access to needed COVID-19 vaccines, therapeutics, and medical devices can be addressed through minor modifications to existing compulsory licensing rules
The European Union suggested that any IP-related access challenges arising during COVID-19 could instead be addressed through nominal changes to the TRIPS articles 31 and 31 bis compulsory licensing framework. In particular, the European Union argued that delays arising from the system’s existing country-by-country and product-by-product notification requirements could be overcome by implementing an emergency uniform notification requirement. [37] Under this alternate proposal, members would provide the WTO Secretariat with a single compulsory licensing notice outlining all vaccines and recipient countries that they planned on supplying under compulsory license, thus reducing alleged administrative burdens to compulsory licensing faced by members in support of the waiver.
3. A TRIPS waiver would undermine existing voluntary licensing partnerships
Several members emphasized that a TRIPS waiver would do more harm than good by destabilizing the international IP framework, and in doing so, jeopardize existing voluntary licensing partnerships between patent holders and third-party manufacturers. In particular, Switzerland highlighted the importance of a “safe regulatory framework” that is “predictable and accountable,” and argued that the TRIPS waiver risked the efforts of the 300+ international partnerships currently working to build production capacity. [38] The need for legal stability to ensure productive and effective technology transfer between originator and generic manufacturers was also underscored.
4. A TRIPS waiver would undermine the development of domestic health technology industries
Several WTO members cited concerns that a TRIPS waiver would undermine innovation in the pharmaceutical sector, thus harming the development of their local industries. For example, while Chile acknowledged that “[the protection of] IP is not an end in itself,” it nonetheless viewed IP as an important tool for development. [39] Similarly, during early TRIPS waiver discussions, both Russia and El Salvador emphasized that “promoting and incentivizing innovation as a tool for boosting and accelerating development” was “a top national priority.” [40] As a result, El Salvador found it “difficult to reconcile” the waiver with the domestic development objectives that it had set as a country.” [41]
Civil society organizations
Over 350 civil society groups, including access to medicines groups, HIV/AIDS organizations, global health and global justice alliances, and human rights groups, expressed strong support for the TRIPS waiver, with many further arguing that governments should view the waiver as a minimum first step to securing access to needed COVID-19 vaccines, therapeutics, and medical devices. Statements from these groups were often directly addressed to heads of WTO members, with requests that governments view the adoption of the waiver as an urgent matter. Four major rationales were advanced by these organizations: (1) the TRIPS waiver enables countries to overcome IP-based supply barriers that cannot be adequately addressed through voluntary or compulsory licensing; (2) the TRIPS waiver enables countries to uphold their human rights obligations; (3) the TRIPS waiver is a necessary but insufficient step toward achieving equitable access to health technologies during COVID-19; and (4) corporate profit should not be prioritized over equitable access. Below, each rationale is discussed in further detail.
Civil society organizations endorsed the view that IP obstructs the production and distribution of affordable COVID-19 health technologies. Many noted that relying solely on voluntary licensing is not a sufficient remedy, as historically it has “failed to leverage global expertise and capacity to scale up manufacturing and deliver equitable access.” [42] Furthermore, the existing compulsory licensing mechanism designed to lawfully circumvent these restrictions was seen to suffer from scaling issues. Many argued that countries are obliged to issue compulsory licenses on a country-by-country and product-by-product basis, and thus that the existing compulsory licensing system is ill-suited for rapid global distribution. It was asserted by many that addressing international access concerns through compulsory licenses “would create a monumental coordination crisis because of the possible need to initiate and win compulsory licensing proceedings in multiple jurisdictions.” [43] Groups highlighted that LMICs have been historically “discouraged from using compulsory licensing for access to medicines due to pressures from their trading partners and pharmaceutical corporations” and that the article 31 bis compulsory licensing pathway has been successfully employed only once, to import patented pharmaceuticals to Rwanda. [44] The TRIPS waiver was promoted as a solution to overcoming these IP-related issues at a global scale necessary to addressing an international pandemic.
2. A TRIPS waiver enables states to uphold their international human rights obligations
Many civil society organizations emphasized the role of a TRIPS waiver in ensuring equal access to critical health technologies consistent with the human rights to health, to receiving and imparting information, to education, to participating in cultural life, and to equally benefitting from scientific progress. In a letter signed by 107 groups, governments were urged to recognize the inequalities exacerbated by the COVID-19 pandemic, with an emphasis on the resulting unequal access to vital technological knowledge among countries. [45] These groups emphasized the importance of ensuring that essential COVID-19 research is made available immediately and everywhere, and argued that “removing legal barriers to knowledge is… needed for the massive, urgent scale-up of vaccine production.” [46] Others echoed WHO Director-General Tedros Ghebreyesus’s 2021 statement that “profits and patents must come second to the human right to health” in supporting arguments that COVID-19 vaccines, as the “common property of humanity,” must be made available as a matter of human rights. [47] The role of the TRIPS waiver as a tool for redressing global inequalities in access to COVID-related health technologies was asserted in most statements.
3. The TRIPS waiver is necessary but insufficient for securing equitable access
Several civil society organizations framed the TRIPS waiver as the necessary but insufficient first of a series of measures that governments must take to ensure equitable access to lifesaving COVID-19 health technologies. [48] In addition, these groups advocated for know-how and technology transfer from patent holders to manufacturers in the Global South, increased direct investment into the expansion of manufacturing capacity in the Global South, and equitable dose sharing from the Global North to the Global South. [49] After the release of the amended waiver in June 2022, over 200 civil society organizations expressed dissatisfaction with the draft ministerial decision and the insufficiency of its application solely to COVID-19 vaccines, its exclusion of some of the world’s largest producers of medical tools, and its restriction of “the free movement and rapid distribution of needed medical products.” [50]
4. Moral appeal: corporate profits should not be prioritized over equitable access
Many civil society organizations adopted the moral position that governments should not prioritize the financial needs of the pharmaceutical industry over the immediate health of humans in need. Emphasis was placed on the collective state responsibility for human life, as well as the priority of this responsibility over states’ competing responsibilities to honor corporate monopolies. [51]
Research-based pharmaceutical companies
Approximately 48.5% of pharmaceutical companies expressed opposition to the proposed TRIPS waiver, either by directly authoring statements or endorsing statements authored by industry-wide associations. These included five companies (AstraZeneca, BioNTech, Janssen, Pfizer, and Sanofi) that are currently partnered with COVAX for the purpose of supplying vaccines to LMICs, as well as pharmaceutical manufacturing trade associations Pharmaceutical Research and Manufacturers of America, International Federation of Pharmaceutical Manufacturers and Associations, and European Federation of Pharmaceutical Industries and Associations. Approximately 7.5% of manufacturers (Bharat Biotech, Biological E, CureVac, Gamaleya, and Moderna) released neutral statements about the TRIPS waiver, indicating a willingness to not enforce their own intellectual property rights but refraining from explicitly endorsing the waiver. The remaining 44% did not release statements about the TRIPS waiver.
The primary arguments advanced against the TRIPS waiver were that (1) a TRIPS waiver would not be effective in increasing global supplies since IP is not a barrier to access; (2) a TRIPS waiver would threaten innovation, thus reducing the pharmaceutical industry’s ability to produce lifesaving technologies; (3) a TRIPS waiver would undermine existing partnerships among manufacturers; and (4) a TRIPS waiver would not rapidly rectify vaccination deficits, which is the ultimate goal of the international COVID-19 response. These arguments are presented below.
- IP is not a barrier to access
Almost all pharmaceutical companies refuted the assertion that IP protection has limited access to patented COVID-19 health technologies throughout the pandemic. Instead, focus was placed on trade restrictions, distribution bottlenecks, and raw material scarcity. [52] Manufacturers emphasized the sufficiency of existing manufacturing capacity and supply chains to provide COVID-19 vaccines to the world’s population, stating that “in 2021, more than 40% of these [3 billion] doses are expected to go to middle- and low-income countries. We believe … that in the next 9 to 12 months, there will be more than enough vaccines produced.” [53] Given these assertions, the Pharmaceutical Research and Manufacturers of America and the International Federation of Pharmaceutical Manufacturers and Associations argued that a TRIPS waiver would be not only unnecessary but harmful to existing manufacturer efforts to expand access through voluntary licensing and technology transfer agreements. [54]
2. A TRIPS waiver threatens innovation
Pharmaceutical manufacturers frequently expressed their opposition to the TRIPS waiver on grounds that waiving IP protection would threaten innovation. Premised on the assertion that IP protections enable innovators to earn the returns necessary to finance risky pharmaceutical research and development (R&D), manufacturers asserted that a TRIPS waiver would undermine ongoing efforts to develop health technologies for new COVID-19 variants. [55] Manufacturers also cited the proposed waiver’s broader deleterious effects on scientific innovation at large, with Pfizer’s chairman and CEO releasing a public letter expressing concern that a TRIPS waiver would disincentivize scientific investments to the particular detriment of small, investor-dependent biotech innovators. [56]
Throughout the pandemic, manufacturers of patented COVID-19 vaccines have underscored their efforts to ensure expanded access by entering into voluntary licensing agreements with third-party manufacturers. In implementing a waiver that would enable countries to suddenly cease enforcing domestic intellectual property rights, manufacturers argued that WTO members risked placing these ongoing inter-manufacturer supply agreements at risk. [57] Two key rationales were presented to support the assertion that IP enforcement is a vital component to ongoing voluntary licensing agreements. First, manufacturers argued that voluntary licenses enable patent owners to carefully pick partner manufacturers that are best equipped to produce quality products. [58] Without such oversight in place, it was alleged that the safety and efficacy of produced vaccines would be threatened. [59] Second, manufacturers asserted that a TRIPS waiver would exacerbate raw material shortages, thus undermining ongoing partnerships, as “entities with little or no experience in manufacturing vaccines [would be] likely to chase the very raw materials that [current manufacturers] require to scale production.” [60]
4. A TRIPS waiver is not a sufficiently rapid solution for rectifying international vaccination deficits
Several manufacturers acknowledged the importance of equitable access to vaccines but maintained that the proposed TRIPS waiver would be unable to rapidly rectify existing vaccination deficits. Instead, they asserted that focus should be shifted toward enhancing voluntary technology transfer arrangements, increasing health infrastructure funding, and expanding educational programs to combat vaccine hesitancy. [61] For example, a statement by AstraZeneca’s executive vice president of Europe and Canada emphasized that “the TRIPS process is no quick fix and could take many months—far too late for millions of people in underserved communities”—and advocated for a suite of “urgent response” measures, including not-for-profit pricing commitments by manufacturers, expanded regional supply chains, and voluntary technology transfer agreements with domestic manufacturers in the Global South. [62]
Stakeholder rationales align with historic divides on the relationship between IP and access to medicines
Among the 131 WTO members that expressed a definite position with respect to the TRIPS waiver, we found that approximately 73% support the waiver (with 65 of 96 supporters endorsing the waiver as co-sponsors). Over 350 civil society groups overwhelmingly aligned with those WTO members in support of the waiver. By contrast, approximately 86% (30 out of 35) pharmaceutical industry stakeholders who issued or endorsed statements regarding the TRIPS waiver uniformly aligned with those WTO members opposed to the waiver.
WTO members opposed to the TRIPS waiver shared overlapping arguments with pharmaceutical industry stakeholders more frequently than endorsing WTO members did with civil society organizations (see Tables 2 and 3). The WTO members that were the most vocally opposed to the TRIPS waiver (the United Kingdom, European Union, and Switzerland) and whose arguments aligned most strongly with pharmaceutical industry stakeholders were also those members in which COVID-19 vaccine manufacturers (AstraZeneca, BioNTech/Pfizer, and Moderna) maintain headquarters or major manufacturing facilities. By contrast, the WTO members that most frequently expressed support for the TRIPS waiver in alignment with civil society-backed rationales were those countries with large domestic generic manufacturing capacities (e.g., India) or that had previously considered or engaged in compulsory licensing for pharmaceuticals (e.g., South Africa, Malaysia, and Sri Lanka). This aligns with the view that in the context of the WTO, an institution primarily designed to facilitate the commercial exchange of goods and services between countries, many members’ decisions to support health-related proposals likely remain highly dependent on prevailing domestic economic priorities.
Notably, several dominant rationales offered by WTO members, pharmaceutical stakeholders, and civil society organizations reflect the same arguments raised during the HIV/AIDS crisis in the early 2000s surrounding the use of compulsory licenses to expand access to antiretrovirals. [63] At the time, pro-compulsory licensing advocates frequently appealed to states’ humanitarian obligations and emphasized the importance of protecting human lives over private profits, while pro-IP advocates rooted their position on grounds of recouping R&D costs, promoting innovation, and securing product quality. [64] The continued use of this language in the context of TRIPS waiver discussions—and the upending endorsement of compulsory licensing by TRIPS waiver opponents as a more feasible solution than the waiver—suggests that the relationship between IP and access to medicine remains highly contentious within the trade and health landscape despite the 2001 Doha Declaration on the TRIPS Agreement and Public Health. [65] Since the WTO operates as a consensus-based decision-making body, this continued division between members presents as a key policy obstacle for states seeking to leverage the international trade system to promote expanded access to health technologies within their domestic health systems.
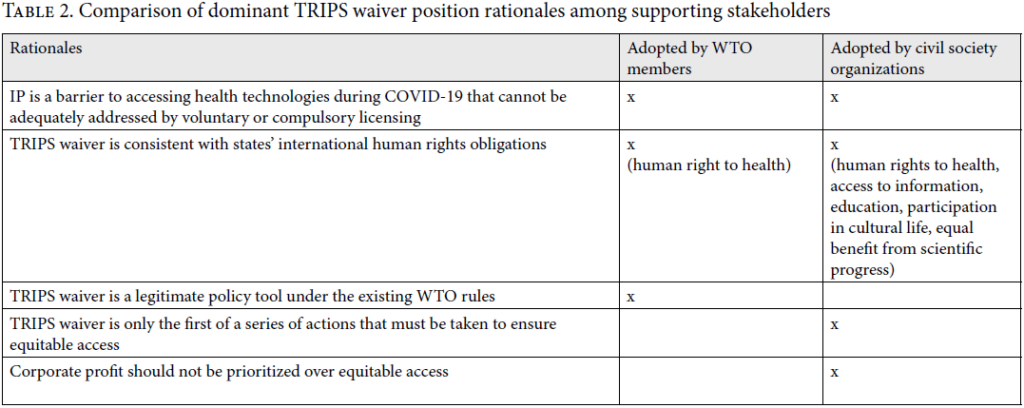
The TRIPS waiver and human rights
Among all stakeholders, the importance of ensuring equitable access to COVID-19 health technologies has not been refuted. In TRIPS waiver discussions among WTO members, differences in vaccine prices and availability between high-income countries and LMICs were frequently highlighted by members as evidence of ongoing inequalities. For example, South Africa argued that it had been charged US$5.25 per dose for AstraZeneca’s vaccine while European Union members had been charged only US$3.50. [66] Several LMIC members also expressed frustration with the unavailability of vaccines for their own populations due to the bilateral supply deals negotiated in advance between high-income countries and manufacturers.
To varying degrees, all WTO members, civil society organizations, and pharmaceutical industry stakeholders that authored or endorsed statements related to the TRIPS waiver acknowledged the importance of rectifying the asymmetric distribution of COVID-19 vaccines between high-income countries and LMICs. However, only civil society organizations consistently framed this inequality in terms of explicit human rights considerations. Here, inequitable international COVID-19 vaccine deployment was frequently viewed as a violation of the human rights to health and to benefit from scientific progress, enshrined in articles 12 and 15 of the ICESCR. The TRIPS waiver was thus supported as an urgent measure explicitly required to rectify ongoing human rights violations. By contrast, WTO members largely refrained from employing human rights language during TRIPS waiver discussions, with the sole reference to article 12 of the ICESCR found in the preambular text of TRIPS waiver sponsors’ September 2021 position summary document. [67] WTO members opposed to the TRIPS waiver often couched their positions in terms of equitable vaccine access, citing either the independent or combined sufficiency of COVAX and inter-manufacturer voluntary licensing agreements in attaining this goal. Similarly, pharmaceutical manufacturers frequently underscored their post-scaling ability to supply vaccines to LMICs that were previously unable to secure doses at the beginning of the pandemic. Thus, while not always framed in terms of explicit human rights obligations, ensuring equitable international access to COVID-19 vaccines has been recognized as a desirable objective by TRIPS waiver proponents and opponents alike—with the efficacy of the proposed TRIPS waiver in successfully achieving this goal at issue.
Given members’ polarizing positions with respect to the TRIPS waiver and the WTO requirement that resolutions be passed through consensus, it is perhaps unsurprising that TRIPS waiver negotiations have struggled to advance. In an attempt to broker a compromise acceptable to all WTO members, the March 2022 TRIPS waiver solution proposed by the European Union, India, South Africa, and the United States sought to make the TRIPS waiver more palatable to opposing members while still providing members with a more streamlined alternative to the compulsory licensing system in articles 31 and 31 bis of the TRIPS Agreement. [68] Public responses to this proposal were largely critical. Civil society organizations decried the compromise as a partial measure incapable of meaningfully increasing access to COVID-19 health technologies and legally unprecedented in its narrow interpretation of the existing article 31 compulsory licensing regime. [69] Conversely, COVID-19 vaccine manufacturers emphasized the compromise’s lack of necessity given recent reports of vaccine overproduction and global demand reductions. [70] Comparable reactions were also elicited from these groups in response to the narrower June 2022 draft decision text. Notably, while civil society groups and pharmaceutical stakeholders alike demonstrated a strong reluctance to endorse compromises that deviated significantly from their original positions, the June 2022 compromise required many WTO members to endorse positions that they initially opposed. While this indicates that the rules-based international trading system remains capable of encouraging consensus-building among its members, the two years of debate preceding this decision suggest that trade-based public health measures are likely ill-suited as first-line responses to urgent and international public health crises.
Access to lifesaving health technologies, such as COVID-19 vaccines, remains starkly inequitable between countries. Reponses by WTO members, civil society organizations, and pharmaceutical industry stakeholders to the proposed TRIPS waiver highlight universal acknowledgment of this unequal health impact of the COVID-19 pandemic. However, where proponents view the TRIPS waiver as a necessary first step toward eliminating IP-driven barriers to access during the pandemic, opponents largely assert that the TRIPS waiver is a political distraction that is both unnecessary and incapable of rapidly expanding the supply of COVID-19 health technologies.
Discourse surrounding the TRIPS waiver suggests that the global community seems to be expressing similar IP and public health arguments as those advanced during the HIV/AIDS crisis. This underscores the continued lack of reliability that countries face when looking to the international trading system as a means to advance public health imperatives and improve access to lifesaving health products. It also suggests the need for deep structural change and how lessons learned are often forgotten.
As WTO members consider the adoption of an expanded TRIPS waiver, the COVID-19 pandemic continues to spread globally with new emerging variants. Without improved international coordination, transparency, and consideration for the health and human rights of all global citizens, states risk remaining ill-prepared for future pandemics and global emergencies. Governments must therefore continue to collectively strive toward the development of equitable solutions so that meaningful progress can be made to improve global access to essential health products. Without decisive action, countries risk being unprepared for future public health crises and continuing to propagate patterns of health inequity.
Acknowledgments
We are grateful for the feedback received from Sharifah Sekalala, Katrina Perhudoff, Lisa Forman, and others during the Connaught Global Challenge Research Program’s “Advancing Rights-Based Access to COVID-19 Vaccines as Part of Universal Health Coverage” paper development workshop on May 17, 2022.
We thank the Connaught Global Challenge Award for funding for this research through the “Advancing Anti-Corruption, Transparency and Accountability Mechanisms to Tackle Corruption in the Pharmaceutical System” and the “Advancing Rights-Based Access to COVID-19 Vaccines as Part of Universal Health Coverage” grants.
Ethics approval
Since this research exclusively employed data obtained from public documents, no ethics approval was required.
Jillian Kohler, PhD, is a professor at the University of Toronto Leslie Dan Faculty of Pharmacy, Dalla Lana School of Public Health, and Munk School of Global Affairs and Public Policy, Toronto, Canada, and founding director of the World Health Organization Collaborating Centre for Governance, Accountability and Transparency in the Pharmaceutical Sector.
Anna Wong is a JD candidate at the University of Toronto Faculty of Law, Toronto, Canada, and research associate at the World Health Organization Collaborating Centre for Governance, Accountability and Transparency in the Pharmaceutical Sector.
Lauren Tailor, MPH, PharmD, is a PhD student at the University of Toronto Dalla Lana School of Public Health, Toronto, Canada, and a research assistant at the World Health Organization Collaborating Centre for Governance, Accountability and Transparency in the Pharmaceutical Sector.
Please address correspondence to Jillian Kohler. Email: [email protected].
Competing interests: None declared.
Copyright © 2022 Kohler, Wong, and Tailor. This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/bync/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction.
[1] International Covenant on Economic, Social and Cultural Rights, G.A. Res. 2200A (XXI) (1966), arts. 12, 15.
[2] World Health Organization, Ten Years in Public Health, 2007–2017 (Geneva: World Health Organization, 2017).
[3] L. Paremoer, S. Nandi, H. Serag, and F. Baum, “COVID-19 Pandemic and the Social Determinants of Health,” British Medical Journal 372/129 (2021); G. Yamey, P. Garcia, F. Hassan, et al., “It Is Not Too Late to Achieve Global COVID-19 Vaccine Equity,” British Medical Journal 376 (2022); T. Mousavi, S. Nikfar, and M. Abdollahi, “Achieving Equitable Access to Medicines and Health Services: A COVID-19-Time Recalled Matter,” Iranian Journal of Pharmaceutical Research 20/4 (2021); C. Batista, P. Hotez, Y. B. Amor, et al., “The Silent and Dangerous Inequity around Access to COVID-19 Testing: A Call to Action,” EClinicalMedicin e 43 (2022); A. De Bengy Puyvallée and K. T. Storeng, “COVAX, Vaccine Donations and the Politics of Global Vaccine Inequity,” Globalization and Health 18/26 (2022).
[4] G. Yamey et al. (see note 3); E. T. Tagoe, N. Sheikh, A. Morton, et al., “COVID-19 Vaccination in Lower-Middle Income Countries: National Stakeholder Views on Challenges, Barriers, and Potential Solutions,” Frontiers in Public Health 9 (2021); S. Owermohle, “Drug Prices Steadily Rise amid Pandemic, Data Shows,” Politico (July 7, 2020), https://www.politico.com/news/2020/07/07/drug-prices-coronavirus-351729; T. Bollyky and C. Bown, “The Tragedy of Vaccine Nationalism: Only Cooperation Can End the Pandemic,” Foreign Affairs 9/5 (2020); W. Zhang, “Export Restrictions on Medical Supply amidst a Pandemic,” Sidley Austin LLP (February 2021), https://www.sidley.com/en/insights/publications/2021/02/export-restrictions-on-medical-supply-amidst-a-pandemic; J. Feinmann, “COVID-19: Global Vaccine Production Is a Mess and Shortages Are Down to More Than Just Hoarding,” British Medical Journal 375 (2021).
[5] D. Devakumar, G. Shannon, S. S. Bhopal and I. Abubakar, “Racism and Discrimination in COVID-19 Responses,” Lancet 395/10231 (2020).
[6] D. J. Hunter, S. S. Abdool Karim, L. R. Baden, et al., “Addressing Vaccine Inequity: COVID-19 Vaccines as a Global Public Good,” New England Journal of Medicine 386/12 (2022); E. Mathieu, H. Ritchie, E. Ortiz-Ospina, et al., “A Global Database of COVID-19 Vaccinations,” Nature Human Behaviour 5 (2021); Our World in Data, “Coronavirus (COVID-19) Vaccinations,” https://ourworldindata.org/covid-vaccinations; V. Pilkington, S. M. Keestra, and A. Hill, “Global COVID-19 Vaccine Inequity: Failures in the First Year of Distribution and Potential Solutions for the Future,” Frontiers in Public Health 10 (2022).
[7] Agreement on Trade-Related Aspects of Intellectual Property Rights, Marrakesh Agreement Establishing the World Trade Organization, Annex 1C, 1896 U.N.T.S. 299 (1994), art 27.
[8] World Intellectual Property Organization, “Frequently Asked Questions: Patents,” https://www.wipo.int/patents/en/faq_patents.html.
[9] N. S. Jecker and C. A. Atuire, “What’s Yours Is Ours: Waiving Intellectual Property Protections for COVID-19 Caccines,” Journal of Medical Ethics 47 (2021).
[10] E. ‘t Hoen, J. Berger, A. Calmy, and S. Moon, “Driving a Decade of Change: HIV/AIDS, Patents and Access to Medicines for All,” Journal of the International AIDS Society 14 (2011); B. R. Edlin, “Access to Treatment for Hepatitis C Virus Infection: Time to Put Patients First,” Lancet Infectious Diseases 16/9 (2016).
[11] See, for example, Medicines Patent Pool, “VaxPaL,” https://medicinespatentpool.org/what-we-do/vaxpal.
[12] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19, IP/C/W/669 (2020).
[13] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19: Revised Decision Text, IP/C/W/669/Rev.1 (2021).
[14] K. Cullinan, “WTO Head Welcomes Compromise on IP Waiver for COVID Vaccines—But Activists and Pharma Express Dismay,” Health Policy Watch (March 16, 2022), https://healthpolicy-watch.news/wto-head-welcomes-ip-waiver-compromise/; World Trade Organization, “Director-General Okonjo-Iweala Hails Breakthrough on TRIPS COVID-19 Solution,” World Trade Organization (March 16, 2022), https://www.wto.org/english/news_e/news22_e/dgno_16mar22_e.htm.
[15] Cullinan (see note 14).
[16] World Trade Organization, Ministerial Conference Twelfth Session, Draft Ministerial Decision on the TRIPS Agreement, WT/MIN(22)/W/15/Rev.2 (2022).
[18] F. Fischer, D. Torgerson, A. Durnova, and M. Orsini, “Introduction to Critical Policy Studies,” in F. Fischer, D. Torgerson, A. Durnova, and M. Orsini (eds), Handbook of Critical Policy Studies (Cheltenham: Edward Elgar Publishing, 2015).
[19] J. Fereday and E. Muir-Cochrane, “Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development,” International Journal of Qualitative Methods 5/1 (2006).
[20] World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/669 (2021); World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/669/Rev.1 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 15–16 October and 10 December 2020, IP/C/M/96 (2021), World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 15-16 October and 10 December 2020, IP/C/M/96/Add.1 (2021), World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/672 (2021); World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/671 (2021); World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/673 (2021); World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/674 (2021); World Trade Organization, General Council, Minutes of Meeting on 16–18 December 2020, WT/GC/M/188 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 23 February 2021, IP/C/M/97 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 23 February 2021, IP/C/M/97/Add.1 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 10–11 March 2021, IP/C/M/98 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 10–11 March 2021, IP/C/M/98/Add.1 (2021); World Trade Organization, General Council, Minutes of Meeting on 1–2 and 4 March 2021, WT/GC/M/190 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 30 April 2021, IP/C/M/99 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 30 April 2021, IP/C/M/99/Add.1 (2021); World Trade Organization, General Council, Minutes of Meeting on 5–6 May 2021, WT/GC/M/191 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 8, 9, and 29 June 2021, IP/C/M/100 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 8, 9, and 29 June 2021, IP/C/M/100/Add.1 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 20 July 2021, IP/C/M/101 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 20 July 2021, IP/C/M/101/Add.1 (2021); World Trade Organization, General Council, Minutes of Meeting on 27–28 July 2021, WT/GC/M/192 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 4 October 2021, IP/C/M/102 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 13–14 October, 5, 18, and 29 November, and 16 December 2021, IP/C/M/103 (2021); World Trade Organization, General Council, Minutes of Meeting on 7–8 October 2021, WT/GC/M/193 (2021); World Trade Organization, General Council, Minutes of Meeting on 15 December 2021, WT/GC/M/195 (2021); World Trade Organization, General Council, COVID-19 and Beyond: Trade and Health, JOB/GC/251/Rev.1 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Draft General Council Declaration on the TRIPS Agreement and Public Health in the Circumstances of a Pandemic, IP/C/W/681 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Urgent Trade Policy Responses to the COVID-19 Crisis: Intellectual Property, IP/C/W/680 (2021); World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19, IP/C/W/684 (2021); World Trade Organization, General Council, COVID-19 and Beyond: Trade and Health, WT/GC/W/823/Rev.6 (2021).
[21] Médecins Sans Frontières, “Countries Obstructing COVID-19 Patent Waiver Must Allow Negotiations to Start 2021” (March 9, 2021), https://www.msf.org/countries-obstructing-covid-19-patent-waiver-must-allow-negotiations.
[22] World Trade Organization, “Global Business/Civil Society Response to COVID-19,” https://www.wto.org/english/tratop_e/covid19_e/covid19_business_e.htm; Canadian Centre for Policy Alternatives, “The TRIPS COVID-19 Waiver,” https://policyalternatives.ca/newsroom/updates/trips-covid-19-waiver.
[23] UNICEF, “COVID-19 Vaccine Market Dashboard,” https://www.unicef.org/supply/covid-19-vaccine-market-dashboard; E. Sagonowsky, “The Top 20 Pharma Companies by 2020 Revenue” Fierce Pharma (March 29, 2021), https://www.fiercepharma.com/special-report/top-20-pharma-companies-by-2020-revenue.
[24] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 10–11 March 2021, IP/C/M/98/Add.1 (2021, see note 20).
[25] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 30 April 2021, IP/C/M/99/Add.1 (2021, see note 20).
[27] World Trade Organization General Council, Minutes of Meeting on 7–8 October 2021, WT/GC/M/193 (2021, see note 20).
[28] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 23 February 2021, IP/C/M/97/Add.1 (2021, see note 20).
[29] World Trade Organization, Council for Trade Related-Aspects of Intellectual Property Rights, Waiver from Certain Provision of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19, IP/C/W/684 (2021, see note 20).
[32] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 10–11 March 2021, IP/C/M/98/Add.1 (2021, see note 20).
[33] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 15–16 October and 10 December 2020, IP/C/M/96/Add.1 (2021, see note 20).
[34] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 10–11 March 2021, IP/C/M/98/Add.1 (2021, see note 20).
[35] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 15–16 October and 10 December 2020, IP/C/M/96/Add.1 (2021, see note 20).
[36] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 23 February 2021, IP/C/M/97/Add.1 (2021, see note 20).
[37] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Urgent Trade Policy Responses to the COVID-19 Crisis: Intellectual Property, IP/C/W/680 (2021, see note 20).
[38] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 23 February 2021, IP/C/M/97/Add.1 (2021, see note 20); World Trade Organization, General Council, Minutes of Meeting on 27–28 July 2021, WT/GC/M/192 (2021, see note 20).
[39] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 8, 9, and 29 June 2021, IP/C/M/100/Add.1 (2021, see note 20).
[40] Ibid.; World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 15-16 October and 10 December 2020, IP/C/M/96/Add.1 (2021, see note 20).
[41] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights Minutes of Meeting on 15–16 October and 10 December 2020, IP/C/M/96/Add.1 (2021, see note 20).
[42] J. Whattam, “It’s Time for Canada to Support the WTO TRIPS Waiver,” MonitorMag (May 6, 2021), https://monitormag.ca/articles/its-time-for-canada-to-support-the-wto-trips-waiver.
[43] Médecins Sans Frontières, “Compulsory Licenses, the TRIPS Waiver and Access to COVID-19 Medical Technologies” (2021), https://msfaccess.org/sites/default/files/2021-05/COVID_TechBrief_MSF_AC_IP_CompulsoryLicensesTRIPSWaiver_ENG_21May2021_0.pdf.
[44] Ibid.; Médecins Sans Frontières, “WTO COVID-19 TRIPS Waiver: Doctors Without Borders Canada Briefing Note,” https://www.doctorswithoutborders.ca/sites/default/files/msf_canada_briefer_on_trips_waiver.pdf.
[45] Statement on Copyright and Proposal of a Waiver from Certain Provisions of the Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement for the Prevention, Containment and Treatment of COVID-19, IP/C/W/669 (2021), https://www.wto.org/english/tratop_e/covid19_e/civil_society_vaccines_waiver_e.pdf.
[47] World Health Organization, “Director-General’s Opening Remarks at the 2021 SADC Seminar on TRIPS Waiver” (2021), https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-2021-sadc-seminar-on-trips-waiver—23-november-2021; Caritas Internationalis, “CI Orientations on COVID-19 Vaccines,” https://www.wto.org/english/tratop_e/covid19_e/caritas_e.pdf.
[48] Statement on Copyright and Proposal of a Waiver from Certain Provisions of the Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement for the Prevention, Containment and Treatment of COVID-19, IP/C/W/669 (2021, see note 45); Canadian Centre for Policy Alternatives, “Civil Society Letter Supporting India’s and South Africa’s Proposal for a TRIPS Agreement Waiver for COVID-19 Treatments” (2021), https://www.policyalternatives.ca/newsroom/updates/civil-society-letter-supporting-indias-and-south-africas-proposal-trips-agreement; Caritas Internationalis, “COVID-19 Vaccines Must Be Made Available for All with Equity and Justice” (2021), https://www.caritas.org/2021/03/vaccinepatents/.
[49] Statement on Copyright and Proposal of a Waiver from Certain Provisions of the Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement for the Prevention, Containment and Treatment of COVID-19, IP/C/W/669 (2021, see note 45); Canadian Centre for Policy Alternatives (2021, see note 48); Caritas Internationalis (2021, see note 48).
[50] Médecins Sans Frontières, “Open CSO Letter to WTO Trade Ministers: Do Not Accept the Current Draft, Demand a Real Waiver” (2022), https://msfaccess.org/open-cso-letter-wto-trade-ministers-do-not-accept-current-draft-demand-real-waiver; Médecins Sans Frontières, “CSO Letter to EU on the Reported Draft Text of the TRIPS Waiver Negotiation” (2022), https://msfaccess.org/cso-letter-eu-reported-draft-text-trips-waiver-negotiation.
[51] Canadian Centre for Policy Alternatives, “Civil Society Letter Supporting India’s and South Africa’s Proposal for a TRIPS Agreement Waiver for COVID-19 Treatments” (2021, see note 48).
[52] A. Bourla, “An Open Letter from Pfizer Chairman and CEO to Colleagues,” Pfizer (2021), https://www.pfizer.com/news/articles/why_pfizer_opposes_the_trips_intellectual_property_waiver_for_covid_19_vaccines; L. Burger and S. Nebehay, “Drugmakers Say Biden Misguided over Vaccine Patent Waiver,” Reuters (May 6, 2021), https://www.reuters.com/business/healthcare-pharmaceuticals/pharmaceutical-association-says-biden-move-covid-19-vaccine-patent-wrong-answer-2021-05-05/; International Federation of Pharmaceutical Manufacturers and Associations, “IFPMA Statement on WTO TRIPS Intellectual Property Waiver” (2021), https://www.ifpma.org/resource-centre/ifpma-statement-on-wto-trips-intellectual-property-waiver/; International Federation of Pharmaceutical Manufacturers and Associations, “IFPMA Statement on TRIPS Discussion Document” (2022), https://www.ifpma.org/resource-centre/statement-ifpma-trips-discussion-document/; PhRMA, “PhRMA Letter to President Joseph Biden 2021” (2022), https://patentdocs.typepad.com/files/2021-03-05-phrma-letter.pdf.
[53] F. Jordans, “Vaccine Maker BioNTech Says No Need to Waive Patents,” ABC News (May 10, 2021), https://abcnews.go.com/Business/wireStory/vaccine-maker-biontech-waive-patents-77601343.
[54] International Federation of Pharmaceutical Manufacturers and Associations (2021, see note 52); PhRMA (see note 52).
[55] Bourla (see note 52); Burger and Nebehay (see note 52); International Federation of Pharmaceutical Manufacturers and Associations (2021, see note 52); International Federation of Pharmaceutical Manufacturers and Associations (2022, see note 52); PhRMA (see note 52).
[56] Bourla (see note 52).
[57] International Federation of Pharmaceutical Manufacturers and Associations (2021, see note 52); PhRMA (see note 52).
[58] International Federation of Pharmaceutical Manufacturers and Associations (2022, see note 52).
[59] Bourla (see note 52).
[61] B. Eakin, “J&J’s Chief Patent Atty Says COVID IP Waiver Won’t Work,” Law360 (April 22, 2021), https://www.law360.com/articles/1375715/j-j-s-chief-patent-atty-says-covid-ip-waiver-won-t-work.
[62] I. Reić, “Vaccinating the World: Op-Ed by Iskra Reic,” AstraZeneca (July 1, 2021) https://www.astrazeneca.com/media-centre/articles/2021/vaccinating-the-world-op-ed-by-iskra-reic.html.
[63] ‘t Hoen et al. (see note 10); Médecins Sans Frontières, “Patents, Prices and Patients: The Example of HIV/AIDS” (2002), https://www.msf.org/patents-prices-patients-example-hivaids.
[64] J. Harrelson, “TRIPS, Pharmaceutical Patents, and the HIV/AIDS Crisis: Finding the Proper Balance between Intellectual Property Rights and Compassion,” Widener Law Symposium Journal (2001).
[65] A. S. Y. Wong, C. B. Cole, and J. C. Kohler, “Intellectual Property and Access to Medicines: Mapping Public Attitudes toward Pharmaceuticals during the United States-Mexico-Canada Agreement (USMCA) Negotiation Process,” Globalization and Health 17/92 (2021).
[66] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Minutes of Meeting on 10–11 March 2021, IP/C/M/98/Add.1 (2021, see note 20).
[67] World Trade Organization, Council for Trade-Related Aspects of Intellectual Property Rights, Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19, IP/C/W/684 (2021, see note 20).
[68] See A. S. Y. Wong, C. B. Cole, and J. C. Kohler, “TRIPS Flexibilities and Access to Medicines: An Evaluation of the Barriers to Employing Compulsory Licenses for Patented Pharmaceuticals at the WTO,” South Centre Research Series No. 168 (October 2022), https://www.southcentre.int/wp-content/uploads/2022/10/RP168_TRIPS-Flexibilities-and-Access-to-Medicines_EN.pdf.
[69] Cullinan (see note 14); J. Love, “QUAD’s Tentative Agreement on TRIPS and COVID 19” (2022), https://www.keionline.org/37544.
[70] M. Mishra and E. Michael, “J&J pulls COVID Vaccine Sales Forecast Due to Low Demand, Supply Glut,” Reuters (April 19, 2022), https://www.reuters.com/business/johnson-johnson-suspends-sales-forecast-covid-19-vaccine-2022-04-19/.
- Share full article
Advertisement
Supported by
Banned From Russian Airspace, U.S. Airlines Look to Restrict Competitors
Because of the war in Ukraine, U.S. carriers have to take the long way on flights to and from Asia, giving an advantage to foreign rivals flying the same routes.

By Kate Kelly and Mark Walker
WASHINGTON — Unable to fly through Russian airspace because of the war in Ukraine, U.S. airlines are stepping up a lobbying campaign on Capitol Hill and at the White House to address what they say is a growing problem: They are losing business to foreign competitors who can take passengers between the United States and Asia faster and more cheaply.
Effectively banned from the polar routes that save time and fuel between the United States and an array of destinations on the other side of the world, U.S. carriers say they are being forced into an aeronautical version of Twister to get passengers where they want to go without taking undue risks.
They have altered trans-Pacific flight plans to ensure they would have somewhere to land in an emergency, reduced passenger and cargo loads to hold down costs as they fly longer distances, and put on hold more than a dozen planned new routes to Mumbai, Tokyo, Seoul and other cities.
On its route from New Delhi to New York City, American Airlines has been forced to stop flights in Bangor, Maine — an hour and a half short of the mark — on 19 occasions, a person familiar with the recent history said. Those stops, which were typically caused by unfavorable winds or weather that depleted the jet fuel supply and ran out the flight crew’s duty hours, delayed passengers and forced a swap-out of 14 pilots and flight attendants.
Those flights were already operating with dozens of the seats deliberately left unfilled, the person added, because less weight on board was required to make the fuel last as long as possible.
Yet many foreign airlines are not banned from flying over Russia, U.S. airlines and their lobbyists say — and are winning more passengers on routes to and from the United States as a result. Continued access to the shorter and more fuel-efficient routes that Russian airspace provides is giving carriers like Air India, Emirates and China Eastern Airlines an unfair advantage, the industry lobbying group Airlines for America said in a recent presentation on Capitol Hill.
Airlines for America estimated the lost annual market share of U.S. carriers at a collective $2 billion per year.
“Foreign airlines using Russian airspace on flights to and from the U.S. are gaining a significant competitive advantage over U.S. carriers in major markets, including China and India,” the presentation, dated February, said. “This situation is directly to the benefit of foreign airlines and at the expense of the United States as a whole, with fewer connections to key markets, fewer high paying airline jobs” and a dent in the overall economy.
U.S. airlines for years had access to Russian airspace through a series of agreements with Moscow. In exchange for that access, they — and other foreign airlines — paid fees to the Russian government for air traffic control support that amounted to hundreds of millions of dollars per year, according to an airline official and an industry advocate.
But after Russia’s invasion of Ukraine last year prompted government officials in the United States, Britain, Canada and Europe to ban Russian aircraft from flying over their airspace, President Vladimir V. Putin of Russia immediately prohibited the United States and other supporters of Ukraine, including Canada and much of Europe, from flying through his skies.
Now airlines are pressing the White House and Congress to fix the problem by subjecting foreign carriers from nations not already banned from Russian airspace to the same restrictions applied to U.S. airlines, effectively forcing them to fly the same routes as their American competitors.
The Biden administration should “take action to ensure that foreign carriers overflying Russia do not depart, land or transit through U.S. airports,” said Marli Collier, an Airlines for America spokeswoman.
The proposal appears to have gained traction with the Transportation Department, which recently drafted an order that would ban Chinese carriers that fly passengers to the United States from flying through Russian airspace, according to three people who were briefed on the order. The order was presented to a group of Biden administration officials, including members of the national security team, on Monday, two of those people said, and has been under consideration this week along with other proposed policy measures.
Transportation Department officials declined to comment. But national security officials are mindful of the potential diplomatic consequences of steps aimed at a longtime ally like India, or of adding further tension to the already strained relationship with China.
A spokesperson at the State Department, which is involved in an interagency government review of the airspace issues, said the department was aware of the concerns and regards the safety of U.S. citizens on foreign soil as a top priority.
“It’s just unfortunate for our air carriers that this has been a collateral issue,” said Manisha Singh, a former assistant secretary for the bureau of economic and business affairs at the State Department who now runs a consulting firm in Washington. “I think we should do anything we can,” she added, noting that the United States should “be careful” before taking steps that might offend foreign countries and affect U.S. tourism and commerce as a result.
Representatives for Delta, American, and United Airlines, the domestic carriers most involved in the lobbying effort, referred questions to Airlines for America, which praised a recent letter by Senate Foreign Relations Committee members to Secretary of State Antony J. Blinken and Transportation Secretary Pete Buttigieg echoing the group’s talking points.
“When foreign airlines overfly Russian territory, even if they do not expect to land on Russian soil, they run the risk of unplanned diversions in Russia for safety, medical, mechanical or more nefarious reasons,” wrote Senator Bob Menendez, Democrat of New Jersey, the panel’s chairman, and Senator Jim Risch of Idaho, its senior Republican. The State and Transportation Departments have not yet responded to the letter, according to someone who has been briefed on the exchange.
Representatives for Air India declined to comment, and representatives for Emirates and China Eastern did not respond to requests for comment.
Arjun Garg, a former chief counsel and acting deputy administrator of the Federal Aviation Administration, said the Biden administration has the legal authority to remedy the complaints from U.S. carriers.
Mr. Garg said both the safety concerns the airlines have flagged and the way in which the current regulations have disadvantaged them are serious dilemmas.
“The foreign air carriers get the benefit of shorter flight times, lower costs, less fuel consumption, all those kinds of advantages that are shut off for U.S. carriers by order of the U.S. government,” Mr. Garg said.
At a time when U.S. fliers are already fed up with fundamental issues like cramped seats, flight cancellations and a cascade of service fees, access to Russian airspace may not be the most pressing worry. Depending on winds, air traffic and other factors on any given day, on a 14-hour flight, avoiding Russian airspace can mean less than an hour of extra flying time in some cases. But it can also mean more than two hours.
But the cost differential is notable. As of Wednesday, the outbound leg of an April round-trip journey from New York’s Kennedy Airport to New Delhi’s Indira Gandhi Airport cost about $1,500 and was estimated at 13 hours and 40 minutes on Air India, according to Travelocity. The most comparable flight on a U.S. carrier: a $1,740 American Airlines trip with estimated flying time of 14 hours and 55 minutes.
But Airlines for America and the major carriers it represents are also highlighting security concerns for Americans who fly over Russia, even on foreign airlines. And history suggests there is cause for anxiety.
In 2014, a Malaysia Airlines flight was shot down over Ukraine , killing 298 people. A Dutch court later convicted, in absentia, two Russian separatists and a pro-Russia Ukrainian with murder.
In 2021, a Ryanair flight from Greece to Lithuania was diverted to Belarus , a close Kremlin ally, after officials in that country alerted air traffic controllers to a supposed bomb threat on the plane. Their true purpose, U.S. prosecutors said , was to arrest a dissident journalist who was a passenger by inventing a false safety issue. (The journalist, Roman Protasevich, was recently put on trial in Belarus, and the officials who the Justice Department says organized the diversion have been indicted in the United States and charged with conspiracy to commit airline piracy.)
Last year, the American basketball star Brittney Griner was detained at an airport near Moscow and later sentenced to nine years in a penal colony for carrying vape cartridges of hashish oil in her luggage. She was freed in December .
There are also operational challenges stemming from the longer routes being flown by U.S. carriers.
Delta Air Lines has redrawn trans-Pacific flight maps repeatedly to comply with both U.S. regulations and the Russian overflight ban, according to internal documents and two people familiar with the changes.
F.A.A. rules require that for long flights, commercial planes must always be within 180 minutes of a suitable airport in case an emergency landing is needed (with certain aircraft, which Delta flies, it can stretch to 207 minutes).
But without access to Russia as an emergency stop, Delta’s Detroit-to-Shanghai flights are now being forced to fly near obscure Pacific landmasses like Shemya Island southwest of Alaska. And if the tiny Shemya airport is too full to handle an emergency landing, Delta pilots must divert to an even farther-flung airport like the one on Midway Atoll in the middle of the Pacific, these people said — adding up to an hour and 40 minutes and more than 3,000 gallons of fuel to the journey when the closer stops are not available.
“You can sometimes think of it as a little bit of an obstacle course,” said Jim Higgins, an aviation professor at the University of North Dakota who flew as a commercial pilot for seven years. Federal regulation around emergency landings, while well-intentioned, he added, “does increase the operational complexity.”
Hari Kumar contributed reporting from New Delhi, and Keith Bradsher from Beijing. Li You contributed research.
Kate Kelly covers money, influence, and policy as a correspondent in the Washington bureau of the Times. Before that, she spent twenty years covering Wall Street deals, key players and their intersection with politics. She is the author of three books, including "The Education of Brett Kavanaugh." More about Kate Kelly
Mark Walker is an investigative reporter in the Washington bureau. He was part of a team that won a Pulitzer Prize for its coverage of Covid-19 in 2020. He grew up in Savannah, Ga., and graduated from Fort Valley State University. More about Mark Walker

IMAGES
VIDEO
COMMENTS
Discussion on the extension of COVID-19 IP waiver. At MC12, trade ministers adopted the Ministerial Decision on the TRIPS Agreement, which gives members greater scope to take direct action to diversify production of COVID-19 vaccines and to override the exclusive effect of patents through a targeted waiver over the next five years. It addresses specific problems identified during the pandemic ...
At a meeting of the Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) on 30 April 2021, WTO members agreed to continue consideration of the proposal for a temporary waiver of certain TRIPS obligations in response to COVID-19 and tasked the chair to report to the General Council on this decision at its next meeting on 5-6 May. Co-sponsors of the proposal announced they ...
provisions relevant to the WTO "TRIPS waiver" debate for COVID-19 vaccines; (2) an overview of the WTO discussions on proposed TRIPS waivers; (3) a discussion of the provisions of the June 17, 2022, Ministerial Decision on the TRIPS Agreement; and (4) an analysis of key issues for Congress presented by the "TRIPS waiver" developments.
Ultimately, however, the WTO is a member-driven institution, and agreement on a TRIPS waiver will require either consensus, or, if it were to go to a vote, a three-fourths majority in accordance with Article IX of the WTO Agreement. Currently, WTO members supporting the waiver simply don't have the numbers to achieve this. About 123 WTO ...
Members agree on the following clarifications and waiver for eligible Members to authorize the ... means already required to be available under the TRIPS Agreement. (d) Determination of adequate remuneration under Article 31(h) may take account of the ... 4 This includes the remuneration aspects of the WHO-WIPO-WTO Study on Promoting Access to ...
The TRIPS Agreement waiver (officially titled the Waiver from certain provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19) [1] is a joint intervention communication by South Africa and India to the TRIPS council of the World Trade Organization (WTO) on 2 October 2020. [2] [3]
On June 8-9, 2021, the World Trade Organization's (WTO) TRIPS Council will hold their first meeting in the wake of the U.S. Trade Representative (USTR) announcing "the Biden-Harris Administration's support for waiving intellectual property protections for COVID-19 vaccines" under the Agreement on Trade-Related Aspects of Intellectual ...
The WTO has actively participated in this collaborative effort, including through a multilateral leaders' task force on COVID-19 and high level trilateral cooperation with the WHO and WIPO. A range of pro-health policy options and interventions are also available for WTO members under the TRIPS Agreement, as implemented in domestic law.
Twenty months after the first TRIPS waiver proposal was tabled by India and South Africa, the World Trade Organization finally arrived at an agreement on Friday that is at best a distant cousin of ...
The TRIPS waiver refers to a proposal, advanced by the governments of South Africa and India, to the World Trade Organization to waive intellectual property rights protection for technologies needed to prevent, contain, or treat COVID-19 "until widespread vaccination is in place globally, and the majority of the world's population has ...
The TRIPS agreement contains 73 Articles describing various obligations on WTO members as regards the granting and enforcement of intellectual property rights.The original waiver proposal would have provided a clean waiver of 40 Articles in the TRIPS, as regards the manufacturing and supply of any COVID 19 countermeasure.
USTR releases summary of five-month consultation on extending WTO TRIPS decision showing broad divergence of views. WASHINGTON - The Office of the United States Trade Representative today announced support for extending the deadline to decide whether there should be an extension of the World Trade Organization (WTO) Ministerial Decision on the TRIPS Agreement (Ministerial Decision) to cover ...
By Christopher Borges. On June 22, 2022, the World Trade Organization (WTO) approved a waiver of intellectual property (IP) protections for COVID-19 vaccine patents, previously secured under the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS). The WTO is currently considering expanding this waiver to include COVID-19 diagnostics and therapeutics in addition to vaccines.
On 17 June 2022, WTO Members adopted a Ministerial Decision on the TRIPS Agreement, document WT/MIN(22)/30 [WT/L/1141]. This Decision is far removed from the comprehensive TRIPS waiver proposal contained in documents IP/C/W/669 and IP/C/W/669/Rev.1 ("original TRIPS ... WTO Members during a public health emergency and consequently a more ...
WTO member rationales for supporting a TRIPS waiver. WTO members in support of the TRIPS waiver advanced four main arguments: (1) the TRIPS waiver is required to address IP-based barriers to access that the existing voluntary and compulsory licensing system is ill-equipped to manage; (2) the TRIPS waiver is important as a tool for promoting ...
The Russian Federation's Timeline. Following the entry into force of the WTO Agreement on 1 January 1995, and in pursuance of the decision adopted by the WTO General Council on 31 January 1995, the GATT 1947 Working Party was transformed into a WTO Accession Working Party under Article XII of the Marrakesh Agreement Establishing the WTO. II.
On 22 August, 2012 the Russian Federation joined the World Trade Organisation (WTO) and became a signatory to the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) which includes rules governing the enforcement of IP rights, civil and administrative procedures and remedies, provisional measures, customs measures and ...
U.S. airlines for years had access to Russian airspace through a series of agreements with Moscow. ... the outbound leg of an April round-trip journey from New York's Kennedy Airport to New ...
the last 10 years. WTO estimates Russian tourism potential as 47 million international travelers by 2020 (World Tourism Organization (WTO), 2003). According to Rus-sia's Federal State Statistics Service (Rosstat, former Goskomstat), a total of 22.51 million people visited Russia in 2003, or 8.15 million people without counting the